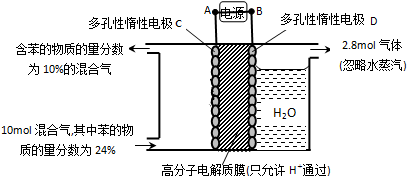
��Ŀ����
13����NAΪ����٤��������ֵ������˵����ȷ���ǣ�������| A�� | ���³�ѹ�£�32g O2��16g O3�Ļ�����У�����Oԭ����ĿΪ2NA | |
| B�� | ��״���£�1mol Na2O ��1mol Na2O2�Ļ�����У�������������Ϊ7NA | |
| C�� | 1mol NaHSO4����ˮ�������ۻ������������������Ŀ��Ϊ2NA | |
| D�� | ��K35ClO3+6H37Cl=KCl+3Cl2��+3H2O�У�������71g Cl2��ת�Ƶĵ�����ĿΪ $\frac{2}{3}$NA |
���� A������n=$\frac{m}{M}$�������ʵ��������Ԫ���غ������ԭ������
B������������������Ϊ���������ӣ�1mol Na2O��1molNa2O2�ж����е����������ӵ����ʵ���Ϊ3mol��
C�����ڵ�NaHSO4�к��е�������ֻ�������ӣ�
D������K35Cl03��H37Cl���ã�����KClO3+6HCl=KCl+3C12��+3H2O��35Cl�õ����ӣ�37Clʧȥ���ӣ�����3molC12����1mol35Cl��
��� �⣺A�����³�ѹ�£�32g O2��O3�Ļ�����к���ԭ����=$\frac{32g}{16g/mol}$��NA=2NA����A��ȷ��
B������������������Ϊ���������ӣ�1mol Na2O��1molNa2O2�Ļ�����к���4mol�����Ӻ�2mol�����ӣ��ܹ�����6mol�������ӣ����е�����������������6NA����B����
C��1mol�����������Ƶ����1mol�����Ӻ�1mol����������ӣ����������������ĿΪ2NA������ˮ����������ӡ������Ӻ���������ӣ�����1mol��������ˮ��Һ�е����������Ϊ3mol����3NA����C����
D���������ClԪ�صĻ��ϼ���+5�۽���Ϊ0���÷�Ӧ��ת�Ƶ�����Ϊ5�����ɵ�������Է�������ԼΪ$\frac{37��5+35}{6}$��2=73.3��������71g Cl2��ת�Ƶĵ�����Ŀ=$\frac{71g}{73.3g/mol}��5$=4.86mol����D����
��ѡA��
���� ���⿼�鰢���ӵ��������йؼ�����жϣ���Ŀ�Ѷ��еȣ����պ������ʵ���Ϊ���ĵĸ���ѧ���밢���ӵ������Ĺ�ϵ��ȷŪ����ӡ�ԭ�ӡ����Ӻ����ʵĹ��ɹ�ϵ�⣮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�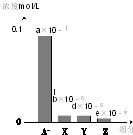 �����£�0.2mol/L��һԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ��a��b��d��e��Ϊ������1����������������˵����ȷ���ǣ�������
�����£�0.2mol/L��һԪ��HA���Ũ�ȵ�NaOH��Һ�������Ϻ�������Һ�в�������ּ�Ũ����ͼ��ʾ��a��b��d��e��Ϊ������1����������������˵����ȷ���ǣ�������| A�� | ����ҺpH=7 | B�� | ����Һ�У�c��A-��+c��Y��=c��Na+�� | ||
| C�� | HAΪǿ�� | D�� | ͼ��X��ʾHA��Y��ʾOH-��Z��ʾH+ |
| A�� | �Ҷ����ͱ�������1��1 | B�� | �Ҵ����Ҷ�����1��2 | ||
| C�� | �״����Ҵ���5��1 | D�� | �״����Ҵ���4��1 |
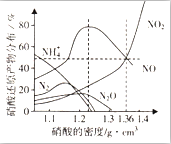 ijЩ�����벻ͬ�ܶȵ����ᷴӦʱ���������ɶ��ֲ�ͬ��̬�Ļ�ԭ�����ͼ������ͬ�����£����ֲ�ͬ�ܶȵ����������Ӧʱ����ԭ����ķֲ�ͼ��ͨ��ͼ���ж�����˵������ȷ���ǣ�������
ijЩ�����벻ͬ�ܶȵ����ᷴӦʱ���������ɶ��ֲ�ͬ��̬�Ļ�ԭ�����ͼ������ͬ�����£����ֲ�ͬ�ܶȵ����������Ӧʱ����ԭ����ķֲ�ͼ��ͨ��ͼ���ж�����˵������ȷ���ǣ�������| A�� | ϡ����������Ӧ��һ����NO���� | |
| B�� | �ܶ�С��1.1g•cm-3������������Ӧ����ԭ����ٷ���������NH4+ | |
| C�� | ij�����Լ�ƿ�ı�ǩע�����ܶ�Ϊ1.26g•cm-3����������50.0%����ȡ���Լ�10ml���100ml��Һ��������ҺpH=1 | |
| D�� | ��������ܶ�Ϊ1.36g•cm-3ʱ�������������ᷴӦ�����ĵ����뱻��ԭ���������ʵ���֮��Ϊ1��1 |
 ԭ���������������X��Y��Z��G��Q��R��T����Ԫ�أ��˵������С��36����֪X��һ��1��2���⻯������м��ЦҼ����Цм���������ԭ�ӹ�ƽ�棻Z��L������2��δ�ɶԵ��ӣ�Qԭ��s�ܼ���p�ܼ���������ȣ�GΪ����Ԫ�أ�R������������ּ���������Ӳ�Ʒ�ĺ��IJ��ϣ�T�������ڱ���ds�����������ֻ��һ�����ӣ�
ԭ���������������X��Y��Z��G��Q��R��T����Ԫ�أ��˵������С��36����֪X��һ��1��2���⻯������м��ЦҼ����Цм���������ԭ�ӹ�ƽ�棻Z��L������2��δ�ɶԵ��ӣ�Qԭ��s�ܼ���p�ܼ���������ȣ�GΪ����Ԫ�أ�R������������ּ���������Ӳ�Ʒ�ĺ��IJ��ϣ�T�������ڱ���ds�����������ֻ��һ�����ӣ���1��Yԭ�Ӻ����7�ֲ�ͬ�˶�״̬�ĵ��ӣ�Tԭ�ӵļ۲��������ʽ3d104s1��
��2����X��Y��Z�γɵ�����ZXY-��XZ2��Ϊ�ȵ����壬��ZXY-��Xԭ�ӵ��ӻ��������Ϊsp��
��3��Z ��R���γɻ�����ף�1mol���к�4mol���ۼ�����������ᷴӦ��������ķ��ӿռ乹�ͷֱ�Ϊ���������Ρ�V��
��4��G��Q��R��������۵����±�������۵�����ԭ��ΪNaF��MgF2Ϊ���Ӿ��壬SiF4Ϊ���Ӿ��壬��SiF4���۵�ͣ�Mg2+�İ뾶��Na+�İ뾶С��������ߣ�������MgF2��NaF����MgF2���۵��NaF��
| ������ | G�ķ����� | Q�ķ����� | R�ķ����� |
| �۵�/K | 993 | 1539 | 183 |
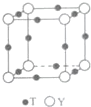
��6��T��Y�γɵľ���ľ����ṹ��ͼ��ʾ��Y���ӵ���λ����6�����辧���ⳤΪacm���ܶ�Ϊ bg•cm-3����٤�������ɱ�ʾΪ$\frac{206}{b{a}^{3}}$���ú�a��b��ʽ�ӱ�ʾ��
��1��FԪ�ؼ۲�����Ų�ʽΪ3d64s4��
��2������B2A2������˵������ȷ���Ǣڢܣ�
��B2A2�е�����ԭ�Ӷ�����8�����ȶ��ṹ
��B2A2���ɼ��Լ��ͷǼ��Լ��γɵķǼ��Է���
��ÿ��B2A2�����ЦҼ��ͦм���Ŀ��Ϊ1��1
��B2A2�����е�A-B������s-sp�Ҽ�
��3��B��C��D����Ԫ�ص�һ�����ܰ��ɴ�С��˳������ΪN��O��C����Ԫ�ط��ű�ʾ����B��C��D����Ԫ������BD2��Ϊ�ȵ�����ķ���ʽΪN2O������Ԫ�ط��ű�ʾ��
��4��A2E��������ԭ�ӵ��ӻ�����Ϊsp3���Ƚ�A2D��A2E���ӵķе㣬���зе�ϸߵ�ԭ��ΪH2O����֮����������Ԫ��D���γ�����ͬ�������壬������ˮ���ܽ�ȸ������O3�������ʽ����
��5��F���ʵľ����ڲ�ͬ�¶��������ֶѻ���ʽ����Ϣ���£�
| ��� | �ѻ���ʽ | �����ⳤ��cm�� |
| �� | ��������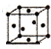 | a |
| �� | ��������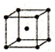 | b |
| A�� | ȡ��ȡҺ����������AgNO3��Һ�а�ɫ��������������ܺ���Cl- | |
| B�� | ȡ��ȡҺ����������Cu��ŨH2SO4���Թܿ��к���ɫ�������������ܺ���NO3- | |
| C�� | ȡ��ȡҺ���������������ữ��BaCl2��Һ���а�ɫ������������һ����SO42- | |
| D�� | �ýྻ�IJ�˿��պȡ��ȡҺ���ھƾ������������գ���ɫ�ʻ�ɫ����һ������Na+ |
������������Һ�м������ᣬ��Ӧ���ң����������ΪpH��ͬ��ϡ���ᣬ��ʼʱ��Ӧ�������Ժ���������ٶȽϿ죬�ٶȱ仯��ԭ���ǣ�������
| A�� | �ݳ�ClO2ʹ������Ũ�Ƚ��� | B�� | ��ʹHClO2�ķֽ���� | ||
| C�� | ��Һ�е�H+������� | D�� | ��Һ�е�Cl-������� |
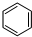 ��g��+3H2��g��$\frac{\underline{\;\;\;\;\;\;\;\;\;����\;\;\;\;\;\;\;\;\;}}{Fe_{3}O_{4}/Al_{2}O_{3}}$
��g��+3H2��g��$\frac{\underline{\;\;\;\;\;\;\;\;\;����\;\;\;\;\;\;\;\;\;}}{Fe_{3}O_{4}/Al_{2}O_{3}}$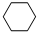 ��g��
��g��