��Ŀ����
���������Դ�����ԭ�������ʡ��������ŷŷ�����ȶ�����ɫ��ѧ�Ļ���Ҫ�����л�ʵ���У���������Ȼ�̼��Һ�������ˮ��Һ������ϡ�������Ũ�����ܽ⡢���������Թ��е���������ˮԡ���ȴ���ֱ���þƾ��Ƽ��ȣ��ܽӴ����������в��á��Ƚ�����������������Ԥ����ԭ��������ˮ���ݹ�ҵ�ϳɰ��з������õ������͵�����ѭ��ʹ�ã���ⷨұ���ƺ�þ��ѡ�����Ȼ��ƺ��Ȼ�þ��������Ӧ�Ľ��������������Ҫ���Ǵ���ɫ��ѧ�Ƕȿ��ǵ���( )
| A���٢� | B���ڢ� | C���ݢ� | D���ۢ� |
A
����

�����ѣ�Ti������Ӳ�ȴ��۵�ߡ�����ʱ����ʴ�����㷺�������¿Ƽ����ϣ�����Ϊ��δ��������������������Ҫ�ɷ�FeTiO3������������Ϊ��Ҫԭ��ұ��������ͬʱ��ø���Ʒ�Ĺ�ҵ�����������£��ش��������⣺
��1���������Ũ���ᷴӦ�IJ���֮һ��TiOSO4����Ӧ�����������ɡ�����Ʒ���׳ơ��̷����仯ѧʽ��________________��
��2���������������м���Feм��Ŀ���� �������ӷ���ʽ��ʾ�������鸱��Ʒ���Ƿ���ʵ�ʵ�鷽���� ��
��3�������������������õ��Ľ������л����������ʣ��ɼ��� �ܽ���ȥ��
��4����Һ���к���Fe2+��TiO2+������Mg2+�������ӡ������£����Ӧ���������Ksp���±���ʾ��
| �������� | Fe��OH)2 | TiO(OH)2 | Mg(OH)2 |
| Ksp | 8.0��10-16 | 1.0��10-29 | 1.8��10-11 |
����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ������д���÷�Ӧ�����ӷ���ʽ��__________________________________________________________________
��5��Mg��ԭTiCl4�����б�����1070K���¶��½��У�����Ϊ��Ӧ���Ƶķ�Ӧ������__________________________
��6����800--1000��ʱ���TiO2Ҳ���Ƶú����ѣ�װ����ͼ��ʾ��ͼ��b�ǵ�Դ��______���������ĵ缫��Ӧʽ________________��

��������к���Cr(OH)3��Al2O3��ZnO��CuO��NiO�����ʣ���ҵ��ͨ�������±��ա���������������Na2Cr2O7�����ʡ�
��֪��ˮ������Һ�д���Na2CrO4��NaAlO2��Na2ZnO2������
��1��ˮ�������Һ��____�ԣ����ᡱ����������С�����
��2������������չ���������Na2CrO4�Ļ�ѧ����ʽ��
____Cr(OH)3+____Na2CO3+_____  = ____Na2CrO4+___CO2+_____
= ____Na2CrO4+___CO2+_____
��3������II����Ҫ�ɷ���Zn(OH)2��___________________________________��
��4����ϵ�в�������Ϊ����������H2SO4��________��ȴ�ᾧ�����ˡ���������H2SO4Ŀ����________________________��
��֪���ٳ�ȥ����II����Һ�д������·�Ӧ��2CrO42��+2H+ Cr2O72��+H2O
Cr2O72��+H2O
��Na2Cr2O7��Na2CrO4�ڲ�ͬ�¶��µ��ܽ�����±�
| �¶� ��ѧʽ | 20�� | 60�� | 100�� |
| Na2SO4 | 19.5 | 45.3 | 42.5 |
| Na2Cr2O7 | 183 | 269 | 415 |
| Na2CrO4 | 84 | 115 | 126 |
��5����ҵ�ϻ�������ˮ�����˺����Һ��Na2CrO4����������H2SO4����ʯī���缫���������������д�����ɸ��ĵ缫��Ӧ����ʽ____________________________��
ij��ѧС����ʵ����ģ�������̿���Ҫ�ɷ�MnO2������Ϊ����ͭ�Ļ�����ȣ��Ʊ��ߴ�̼���̣��������£����ֲ����������ԣ���
�� ��������ƿ�У���ͼ��ͨ�������������С����̡���������Ҫ��Ӧԭ��Ϊ��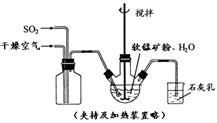
SO2+ H2O = H2SO3
MnO2+ H2SO3 = MnSO4+ H2O
��������������SO2 �ὫFe3+��ԭΪFe2+��
�� ���̡����������ƿ�м���һ������MnO2��ĩ��
�� ����Na2CO3��Һ����pHΪ3.5���ң����ˡ�
�� ������ҺpHΪ6.5��7.2 ������NH4HCO3 ����dz��ɫ�ij������ɣ����ˡ�ϴ�ӡ�����õ��ߴ�̼���̡�
�ش�
��1�������̡���Ӧ�������и�����MnS2O6 �����ɣ��¶ȶԡ����̡���Ӧ��Ӱ������ͼ��Ϊ����MnS2O6�����ɣ������̡��������¶��� ��
��2�����ı�1�����е�pHΪ3.5ʱ��������Ҫ�ɷ��� �����м���һ������MnO2��ĩ����Ҫ������ ����Ӧ��Ӧ�����ӷ���ʽΪ ��
��1��������Ӧ���������pH
| ���� | Fe(OH)3 | Fe(OH)2 | Mn(OH)2 | Cu(OH)2 |
| ��ʼ����pH | 2.7 | 7.6 | 8.3 | 4.7 |
| ��ȫ����pH | 3.7 | 9.6 | 9.8 | 6.7 |
��3���������õ���Һ�к���Cu2+�������ӹ��������ܵ����MnS��ȥCu2+�������ˣ��õ�������MnSO4����ƽ���ƶ�ԭ�����ͼ���MnS������_________________��
��4�����м���NH4HCO3 ������Ӧ�����ӷ���ʽ��____________________��
��5��������г����Ƿ�ϴ�Ӹɾ��ķ����� ��
�������ʵĹ�ҵ�Ʊ�ԭ���ķ���ʽ��д��ȷ����
A���������ƣ�Na2CO3+Ca(OH)2 CaCO3��+2NaOH CaCO3��+2NaOH |
B���Ҵ���C6H12O6 2C2H5OH+2CO2�� 2C2H5OH+2CO2�� |
C����������Cu+2H2SO4��Ũ�� CuSO4+SO2��+2H2O CuSO4+SO2��+2H2O |
D��������Ca(ClO)2+4HCl��Ũ�� CaCl2+2Cl2��+2H2O CaCl2+2Cl2��+2H2O |
���й�ҵ�����У��йع�ϵʽ�������
| A��Ư�ۣ�2Cl2��Ca(ClO)2 | B��H2SO4��FeS2��2H2SO4 |
| C������C��H2��2/3 NH3 | D��HNO3�� NH3��HNO3 |
�������ʵ��Ʊ������Ϲ�ҵ����ʵ�ʵ���( )
| A��������ͨ�����ʯ��ˮ����Ư�� |
| B�������ӽ���Ĥ����ⱥ��ʳ��ˮ�Ʊ��ռ���������� |
| C����������������Ϻ��ȼ��������ˮ�����Ʊ����� |
| D����SO2��O2�Ļ�����Ӹ�ѹ��ͨ���Ӵ��ң��Ʊ�SO3 |
���ڹ�ҵ������������������� (����)��
| A������ͨ��������Ҫԭ���Ǵ��ʯ��ʯ��ʯӢ |
| B�������������ʡ����ᡢ��ε���Ҫԭ�� |
| C������������������������������������������ˮ�����Ƶ�Ũ���� |
| D��������ͨˮ�����Ҫԭ���������ʯ��ʯ |
ij������CaSO4��H2O��NH3��CO2�Ʊ�(NH4)2SO4���乤���������£�
�����ƶϲ��������� (����)��
| A��������ͨCO2������(NH4)2SO4���� |
| B������1 mol(NH4)2SO4��������2 mol NH3 |
| C����ʵ��������CO2�ɱ�ѭ��ʹ�� |
| D��ֱ��������Һ�ܵõ�������(NH4)2SO4 |