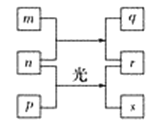
��Ŀ����
����Ŀ����ѧѧϰ���о��벻��Ԫ�����ڱ�����ΪԪ�����ڱ���һ���֣����Т�-��ֱ����һ��Ԫ�ء��ش��������⣺
��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 | |
1 | �� | |||||||
2 | �� | �� | �� | �� | �� | �� | ||
3 | �� | �� | �� |
(1)����Ԫ���У���һ��Ԫ������Ȼ�����γɵ�����������࣬��Ԫ�������ڱ��е�λ����______��
(2)�ࡢ�ᡢ��Ԫ�ص����Ӱ뾶�ɴ�С��˳��Ϊ______(�����ӷ��ű�ʾ)��
(3)д���ɢ١��ޡ�������Ԫ���γɵ�һ�����ӻ�����ĵ���ʽ___________��
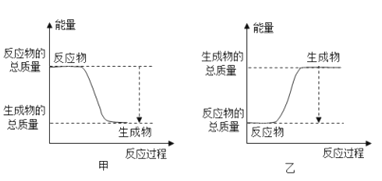
(4)����Ԫ������һ��Ԫ�ص���������������ﶼ�����ԣ�������Ԫ�ص�ԭ�ӽṹʾ��ͼ______�����������£���Ԫ�صĵ����������ܷ�����Ӧ���˷�Ӧ������Ұ�⺸�Ӹֹ죬��÷�Ӧ�������仯����ͼ______��ʾ��![]() ��ס����ҡ�
��ס����ҡ�![]()
�Ӣ�-������ѡԪ�أ�������Ҫ��д��һ���û���Ӧ��_____________________��Ҫ�ǽ�������1 + ������1���ǽ�������2 + ������2
���𰸡��ڶ����ڵ�IVA�� Cl->Na+>Al3+ ![]()
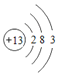 �� 2F2+2H2O =4HF+O2��C+H2O
�� 2F2+2H2O =4HF+O2��C+H2O![]() CO+H2��3Cl2+2NH3=6HCl+N2��4NH3+3O2=6H2O+2N2
CO+H2��3Cl2+2NH3=6HCl+N2��4NH3+3O2=6H2O+2N2
��������
��-��Ԫ��Ϊ��H��Li��Be��C��N��O��F��Na��Al��Cl��
��1����һ��Ԫ������Ȼ�����γɵ�����������࣬ΪC����λ��ӦΪ�ڶ����ڵ�IVA�壻
��2���ࡢ�ᡢ��Ԫ�ص����ӷֱ�Ϊ��Na+��Al3+��Cl-�����Ӳ�Խ������Ӱ뾶Խ����ͬ���Ӳ�ṹ�����ӣ�ԭ������Խ��뾶ԽС��������뾶�ɴ�С��˳��ΪCl��>Na+��Al3+��
��3���١��ޡ�������Ԫ���γɵ�һ�����ӻ�����ΪNaOH�������ʽΪ��![]()
��4��Al����������������ﶼ�����ԣ�Alԭ�ӽṹʾ��ͼΪ��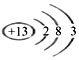 ������Al��Fe2O3Ұ�⺸�Ӹֹ죬��ӦΪ���ȷ�Ӧ���ų��������ȣ���Ӧ�������仯����ͼ�ױ�ʾ��
������Al��Fe2O3Ұ�⺸�Ӹֹ죬��ӦΪ���ȷ�Ӧ���ų��������ȣ���Ӧ�������仯����ͼ�ױ�ʾ��
��5���Ӣ�-������ѡԪ�أ����ǽ�������1 + ������1���ǽ�������2 + ������2��Ҫ��д�����û���Ӧ�У�2F2+2H2O =4HF+O2��C+ H2O![]() CO+H2��3Cl2+2NH3=6HCl+N2 ��4NH3+3O2=6H2O+2N2��
CO+H2��3Cl2+2NH3=6HCl+N2 ��4NH3+3O2=6H2O+2N2��