��Ŀ����
11���ش��������⣺��1�������������������ϩ�����ϩ��������������������������ߵ���BCD��
A��ˮ
B����ˮ
C��������Ȼ�̼��Һ
D���������������Һ
��2����11.2L��״���µļ������ϩ�Ļ������ͨ��������������Ȼ�̼��Һ�г�ַ�Ӧ��������Ȼ�̼��Һ������5.6g����ԭ��������м�������ϩ��������Ϊ6��7��
��3��̼ԭ����Ϊ6�������У���4������-CH3����ͬ���칹����2�֣�д�����к�3������ͬ���칹��Ľṹ��ʽCH3CH��CH3��CH2CH2CH3��CH3CH2CH��CH3��CH2CH3��
���� ��1���������ڱ����������ʽ��ȶ�����ϩ�к���C=C�����ţ������巢���ӳɷ�Ӧ�������Ը��������Һ����������ԭ��Ӧ���ݴ˽����жϣ�
��2����ϩ���巢���ӳɷ�Ӧ��������Ȼ�̼��Һ���ӵ�����Ϊ��ϩ�����������������塢��ϩ�����ʵ��������������������ʵ������ټ��������������������ԭ��������м�������ϩ�������ȣ�
��3�����ݼ�̼����ȡ��������дC6H14��������4��̼ԭ�ӵ�ͬ���칹�壻��������к���3�������������Ϻ���5��C��Ȼ���ƶ���д�������������л���Ľṹ��ʽ��
��� �⣺��1��A���������ϩ����������ˮ��ˮ��������ߣ���A����
B����ϩ���巢���ӳɷ�Ӧʹ��ˮ��ɫ�������鲻��Ӧ��������ˮ���𣬹�B��ȷ��
C����ϩ���巢���ӳɷ�Ӧʹ������Ȼ�̼��Һ��ɫ�������鲻���巴Ӧ������������Ȼ�̼���𣬹�C��ȷ��
D����ϩ�к���C=C�����ţ������Ը��������Һ����������ԭ��Ӧ�����Ը��������Һ��ɫ�������鲻�����Ը��������Һ��Ӧ�����Կ������Ը��������Һ���𣬹�D��ȷ��
�ʴ�Ϊ��BCD��
��2����ϩ���巢���ӳɷ�Ӧ��������Ȼ�̼��Һ���ӵ�����Ϊ5.6g����m��C2H4��=5.6g��n��C2H4��=$\frac{5.6g}{28g/mol}$=0.2mol��
��n��CH4��+n��C2H4��=$\frac{11.2L}{22.4L/mol}$=0.5mol����n��CH4��=0.5mol-0.2mol=0.3mol��m��CH4��=0.3mol��16g/mol=4.8g��
����ԭ��������м�������ϩ������֮��=4.8g��5.6g=6��7��
�ʴ�Ϊ��6��7��
��3������6��C������ΪC6H14��������4��̼ԭ�ӣ�֧��Ϊ2������2����������ͬһ��̼ԭ���ϣ�Ҳ�����ڲ�ͬ��̼ԭ���ϣ���ͬ���칹���У�CH3CH2C��CH3��3��CH3CH��CH3��CH��CH3��CH3���ܹ�����2�֣�����3�������������Ϻ���5��C�����ܵĽṹ��ʽ�У�CH3CH��CH3��CH2CH2CH3��CH3CH2CH��CH3��CH2CH3��
�ʴ�Ϊ��2�� CH3CH��CH3��CH2CH2CH3��CH3CH2CH��CH3��CH2CH3��
���� ���⿼�����л������ʽȷ����ͬ���칹�����д���л���ṹ�����ʵ�֪ʶ����Ŀ�Ѷ��еȣ������漰��֪ʶ��϶࣬��ֿ�����ѧ�����Ӧ�û���֪ʶ��������ע������ͬ���칹��ĸ����дԭ����ȷ�����л���ṹ�����ʣ�

| A�� | NO��NO2 | B�� | CO��CO2 | C�� | CO2��SO2 | D�� | CH4��NH3 |
��֪��
�����������������pH
| Mn��OH��2 | Fe��OH��2 | Fe��OH��3 | Cu��OH��2 | |
| ��ʼ����ʱ | 8.3 | 6.3 | 2.7 | 4.7 |
| ��ȫ����ʱ | 9.8 | 8.3 | 3.7 | 6.7 |
�ش��������⣺
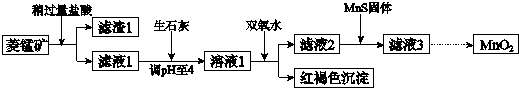
��1�������ʵ����̿�ʹ��ǰ�轫����飬��ҪĿ��������Ӵ��������߷�Ӧ���ʣ������ܽ�MnCO3�Ļ�ѧ����ʽ��MnCO3+2HCl=MnCl2+CO2��+H2O��
��2������Һ1�м���˫��ˮʱ����Ӧ�����ӷ���ʽ��2Fe2++H2O2+4H2O=2Fe��OH��3��+4H+��
��3����Һ2�м����Թ��������ܵ����MnS���Գ�ȥCu2+����Ӧ�����ӷ���ʽ��MnS+Cu2+=Mn2++CuS��
��4����MnCl2ת��ΪMnO2��һ�ַ���������������������������ữ��NaClO3��Һ��MnCl2�������÷�Ӧ�����ӷ���ʽΪ��5Mn2++2ClO3-+4H2O=Cl2��+5MnO2+8H+��
��5����MnCl2ת��ΪMnO2����һ�ַ����ǵ�ⷨ��
������MnO2�ĵ缫��Ӧʽ��Mn2+-2e-+2H2O=MnO2+4H+��
����ֱ�ӵ��MnCl2��Һ������MnO2��ͬʱ���������Cl2������Cl2�IJ����ǽ���ʪ�ĵ��۵⻯����ֽ������������������ֽ������֤����Cl2���ɣ�
����������MnCl2��Һ�м���һ������Mn��NO3��2��ĩ������Cl2��������ԭ�����������������£�����Mn2+Ũ��[������c��Mn2+��/c��Cl-��]��������Mn2+�ŵ磨������Cl-�ŵ磩��
| A�� | �ձ� | B�� | ������ | C�� | ������ƿ | D�� | �Թ� |
| A�� | ԭ�Ӿ�����۵�һ���ȷ��Ӿ���� | |
| B�� | SO2��SO3���ǷǼ��Է��� | |
| C�� | PCl3������P-Cl�ļ���С��109��28? | |
| D�� | �������������ӱ��������� |
| A�� | CH3CHO | B�� | CH3COOC2H5 | C�� | CH3COOH | D�� | CH3CH2OH |
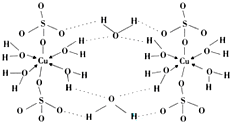 �-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���
�-������ͭ��������Ļ���������Cu4O��PO4��2����ͨ�����з�Ӧ�Ʊ���
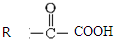 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$ 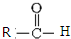 +CO2
+CO2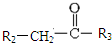 $\stackrel{NaOH��aq������}{��}$
$\stackrel{NaOH��aq������}{��}$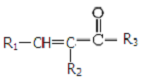 +H2O
+H2O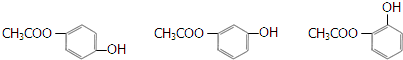 ������֮һ����
������֮һ����