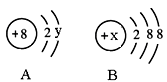��Ŀ����
(1)ͬ��ͬѹ�£�ͬ�����NH3��H2S�������������___________��ͬ������NH3��H2S������������__________��ͬ������NH3��H2S������������ԭ�Ӹ�������___________��������������ԭ�Ӹ�����ȣ����ǵ����ʵ�������________��
(2)Na2SO4??10H2O��Ħ��������__________��483gNa2SO4??10H2O������Na2SO4??10H2O�����ʵ�����_______������Na�������ʵ�����_________������H2O���ӵ���Ŀ��_______����
��ÿ��1�֣���8�֣� ��1��1:2 ��2:1 ��3:1 �� 2:3��2��322g/mol��1.5mol�� 3 mol ��9.03��1024
���������������1�����ݰ����ӵ����ɿ�֪��ͬ��ͬѹ�£�ͬ�����NH3��H2S�������ʵ�����ȣ������������17:34��1:2��ͬ������NH3��H2S������������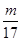 :
: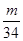 ��2:1��ͬ������NH3��H2S������������ԭ�Ӹ�������
��2:1��ͬ������NH3��H2S������������ԭ�Ӹ�������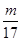 ��3:
��3: 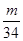 ��2��3:1��������������ԭ�Ӹ�����ȣ�����ݷ���ʽ��֪�����ǵ����ʵ�����2:3��
��2��3:1��������������ԭ�Ӹ�����ȣ�����ݷ���ʽ��֪�����ǵ����ʵ�����2:3��
��2��Na2SO4??10H2O��Ħ�������ǣ�142+180��g/mol��322g/mol ��483gNa2SO4??10H2O������Na2SO4
??10H2O�����ʵ�����483g��322g/mol��1.5mol������Na�������ʵ�����1.5mol��2��3.0mol������H2O���ӵ���Ŀ��1.5mol��10��6.02��1023/mol9.03��1024����
���㣺�������ʵ������йؼ���

 �Ķ��쳵ϵ�д�
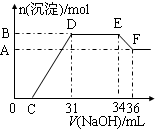
�Ķ��쳵ϵ�д�ʵ����Ҫ0.10mol/LNaOH��Һ470mL��������Һ����������ش��������⣺
��1��ʵ���г���������ƽ���ձ�������������Ͳ��ҩ�����Ҫ�����������У� ��
��2�����ݼ����֪������NaOH������Ϊ g��
��3������ʱ��������ƿ����Һ�İ�Һ��������̶������У��Ǻ�ƿ�������һ�������� ��
��4������ʱ,�������ˮ�����̶��ߣ������ȡ�Ĵ�ʩ�ǣ� ��
��5�����в���������Ũ���к�Ӱ�죨��д��ĸ��
ƫ�͵��� ����Ӱ����� ��
| A������������������룻 |
| B����NaOH����ֽ���ϳ����� |
| C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У� |
| D������ʱ���ӿ̶��� |