��Ŀ����
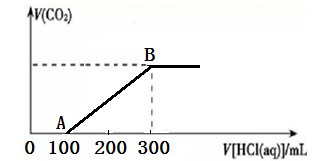
�̷���FeSO4��7H2O���ڻ�ѧ�ϳ���������ԭ������������ҵ�ϳ��÷���м����һ��Ũ�ȵ�������Һ�Ʊ��̷���
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ ����>��<��=��40%��
��2��ʵ��������20%�������ᣨ100�˷������ẬSO3 20�ˣ�����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL����״��������ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200mL2mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS������������������λС������
��1��7.14 mol��L��1 ; > ��2��+2�֣�
��2��0.77��2�֣�
��3��FeSO4��Fe2(SO4)3��10H2O��2�֣�
��4����42��60mL��3�֣��� ��0.33��3�֣�
���������������1����Ũ��������ΪxmL��1.4x��50%/(98*x*0.001)=7.14 mol��L��1����50%��������30%��������ܶȷֱ�ΪA,B�����õ���Һ����������Ϊ(0.5A+0.3B)/(A+B)����ΪA>B���Ը�ʽ����40%��
��2��������������98%Ũ�����м���ͨ��SO3 ,��Ϊ20%ָ��������,20%�ķ�������ɱ�ʾΪH2O��nSO3���Ʊ���Դ������һ��������ΪH2SO4����n-1��SO3���ɸû������H2SO4���ܼ�����SO3�����ʣ���������ϵ�ɵ� 98 ��80��n-1���� ="(1-20%):" 20%�����n="1.3" ���������������б仯�� H2O��1.3SO3��Ϊ1/1.3H2O��SO3����n=0.77��
��3�������������ó��������ᱵ��9.32�˵����ʵ���Ϊ0.04mol,��n(SO42-)=0.04mol��112mL����״�������������ʵ���Ϊ0.005mol,����Cl2---2Fe2+��ϵʽ�����n(Fe2+)=0.01mol��˵��ԭ�����д���FeSO40.01mol������Ϊ1.52g��n(Fe2(SO4)3)=��0.04-0.01��/3=0.01mol������Ϊ4g,��ᾧˮ�����ʵ���Ϊ��7.32-1.52-4��/18=0.1mol�����ߵ����ʵ���֮��Ϊ1��1��10���ʾ���Ļ�ѧʽΪFeSO4��Fe2(SO4)3��10H2O��
��4����200mL2mol/Lϡ������Һ�����ʵ���Ϊ��0.20L��2mol/L=0.4mol�������������ȫ��ΪCu2S����Ҫ����������ʵ���Ϊx��
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
3 16
8.64/160=0.054 x
x=0.288mol,��ʣ����������ʵ�����Ӧ��ʣ�����������ʵ���Ϊ0.4mol-0.228mol=0.112mol��0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O������4H+----3Fe2����ϵʽ�����n(Fe2��)=0.084mol������Ҫ 2 mol/L (NH4)2Fe(SO4)2��Һ�����V =42ml������ͬ�������ټ����������ȫ��ΪCuS����Ҫ��������ʵ���Ϊx��
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
8 3
x 8.64/96=0.09
x=0.24mol,ʣ�����������ʵ���Ϊ0.4mol-0.24mol=0.16mol��0.16mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��NO3-��3Fe2����4H���� NO����3Fe3+��2H2O������4H+----3Fe2����ϵʽ�����n(Fe2��)=0.12mol������Ҫ 2 mol/L (NH4)2Fe(SO4)2��Һ�����V =60ml���ʴ�Ϊ��42��V��60��
����V=48����48mL��NH4��2Fe��SO4��2������Һn(Fe2��)=0.096mol����ȫ��Ӧ��Ҫ��������ʵ���Ϊ��
NO3-��3Fe2����4H���� NO����3Fe3+��2H2O 1mol 5mol
0.096 0.128
������������ʵ���Ϊ0.128mol������������ﷴӦ����������ʵ���Ϊ0.4mol-0.128mol=0.272mol����Cu2S�����ʵ���Ϊx��CuS�����ʵ���Ϊy��160x+96y=8.64g.��
10NO3-��3Cu2S��16H����6Cu2����10NO����3SO42-��8H2O
x 16x/3
8NO3-��3CuS��8H���� 3Cu2����3 SO42-��8NO��+ 4H2O
y 8y/3
16x/3+8y/3=0.272mol ,���y=0.03
��������CuS������2.88g,������������=2.88/8.64=0.33�𣺻������CuS����������Ϊ33%��
���㣺������Ҫ������ݷ���ʽ�����йؼ��㣬�ѶȽϴ�ע����������ʱ���ü��������ݼ����������ȡֵ��Χ��

����98%��ŨH2SO4��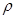 =1��84g/cm3�����Ƴ�Ũ��Ϊ0��5mol/L��ϡ����500mL��
=1��84g/cm3�����Ƴ�Ũ��Ϊ0��5mol/L��ϡ����500mL��
��1���뽫���в�������ȷ��������ں����ϣ�
| A������Ͳ��ȡŨH2SO4 |
| B�������ߵ�ҡ�� |
| C���ý�ͷ�ιܼ�ˮ���̶� |
| D��ϴ���ձ��ڱںͲ�����������ϴҺת������ƿ |
F������Һת������ƿ
�������ȷ˳��Ϊ_____________
��2����Ҫ�ش��������⣺
������ŨH2SO4�����Ϊ________mL��
�����ʵ������15mL, 20mL, 25mL����Ͳ��ѡ��_______mL����Ͳ��á���ȡʱ������Ͳ���ɾ�����ˮϴ����ֱ����ȡ��ʹʵ�����ս��______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�۽�ŨH2SO4���ձ��ڱ�����ע��ʢˮ���ձ��У����Ͻ����Ŀ����_______���������������Һ�彦������ʹ���ս��_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
����ת������ƿǰ�ձ���Һ��Ӧ_______�������ʹŨ��_______��ϴ���ձ�2��3�Σ�ϴҺҲҪת������ƿ�������ʹ���ս��_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�ݶ���ʱ����ʹ��Һ��Һ����̶������У������ӻ�ʹ���_______�����ӻ�ʹ���_______���ƫ�ߡ�����ƫ�͡�����Ӱ�족����


 ��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�
��4�������������ж���������Σ���ܴ���˺�����ˮ������д��������ŷţ�


 Al2��SO4��3��
Al2��SO4��3��