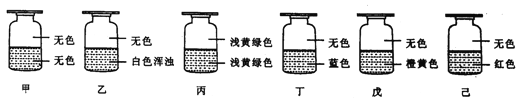
��Ŀ����
����Ŀ����������(NaClO2)����ǿ�����ԣ������ֽ⣬����Ư����ʳƷ�������ȡ�
��.�������Ƶ��Ʊ�
�������Ƶ�Ϊԭ���Ʊ��������ƵĹ����������£�
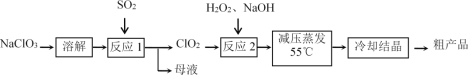
(1)����Ӧ1���е���������_____(�ѧʽ����ͬ)��ĸҺ�����ʵ���Ҫ�ɷ���_____��
(2)ÿ��1mol SO2�μӷ�Ӧ�������Ͽ�����ClO2�����ʵ���Ϊ________mol��
(3)�����SO2��ΪNa2SO3��ϡH2SO4��������Ӧ1�������ӷ���ʽΪ________��
(4)����Ӧ2��ʹ��H2O2����ʹ���������ʵ���Ҫ������____________________��
��.�������Ƶ�Ӧ��
(5)������������ˮ��������ˮ�п��ܲ��������������ƣ����Լ�������������ȥ�������������Σ����������������������Ʒ�Ӧ�⣬��������������____________��
(6)ʵ���ҿ����������ƺ������������������·�Ӧ�Ʊ�ClO2���塣
��������Ӧ�����ӷ���ʽΪ____________��
��ClO2Ҳ������ɱ�������������������ǵ����ʵ���Cl2��_______����
���𰸡�NaClO3 Na2SO4 2 2ClO3��+SO32��+2H+=2ClO2��+SO42��+H2O �����H2O2�ɷֽ�Ϊ������ˮ���������������� Fe2+������ΪFe3+��Fe3+ˮ������Fe(OH)3��Fe(OH)3���н�������ʣ���������ˮ������ ClO2��+ClO3��+2H+=2ClO2��+H2O 2.5
��������
���������Ƶ�Ϊԭ���Ʊ��������ƵĹ��������У���Ӧ���з�Ӧ��ΪNaClO3��SO2����֪������ΪClO2��ClԪ�صĻ��ϼ���+5��Ϊ+4��NaClO3������������SO2Ϊ��ԭ�������Ӧ��������ΪSO42-�������Ӧ��Ļ�ѧ����ʽΪ2NaClO3+SO2=2ClO2+Na2SO4����Ӧ���з�Ӧ��ΪClO2��H2O2��NaOH����֪����ΪNaClO2��ClԪ�صĻ��ϼ���+4��Ϊ+3��ClO2������������H2O2����ԭ�������������ԭ��Ӧԭ��֪2ClO2+ H2O2+2NaOH= 2NaClO2+O2+2H2O��
(1) ��Ӧ���з�Ӧ��ΪNaClO3��SO2����֪������ΪClO2��ClԪ�صĻ��ϼ���+5��Ϊ+4��NaClO3�������������Ϸ�����Ӧ��Ļ�ѧ����ʽΪ2NaClO3+SO2=2ClO2+Na2SO4 �����ݸ÷�Ӧ�ò����Ӧ���ĸҺ�����ʵ���Ҫ�ɷ���Na2SO4��
(2)��Ϸ�Ӧ��Ļ�ѧ����ʽΪ2NaClO3+SO2=2ClO2+Na2SO4 ���۲컯ѧ������֪ÿ��1mol SO2�μӷ�Ӧ�������Ͽ�����ClO2�����ʵ���Ϊ2mol��
(3)�����SO2��ΪNa2SO3��ϡH2SO4��NaClO3��ClO3����������������������ClO2��SO32������ԭ������������SO42�������ȱ����ƽ֪����Ӧ1�������ӷ���ʽΪ2ClO3��+SO32��+2H+=2ClO2��+SO42��+H2O��
(4)Ϊ��ʹClO2�����ת��ΪNaClO2����Ҫ���������H2O2���������Լ����ȥ������H2O2�ֽ�Ϊ������ˮ����������Ӧ2��ʹ��H2O2����ʹ���������ʵ���Ҫ�����Ƕ����H2O2�ɷֽ�Ϊ������ˮ���������������ʣ�
(5)�������������������Ʒ�Ӧ�����ӷ���ʽΪ4Fe2++ClO2��+4H+ = 4Fe3++Cl��+2H2O��Fe2+������ΪFe3+��Fe3+�ᷢ��ˮ�⣬�䷽��ʽΪFe3++3H2O Fe(OH)3(����)+3H+��Fe(OH)3���н�������ʣ���������ˮ�����ʣ���������������������������Ʒ�Ӧ�⣬�����������ã�
(6) ���������ƺ������������������·�Ӧ�Ʊ�ClO2���壬�䷴Ӧ��Ϊ�������ƺ������ƣ�����ΪClO2��ClԪ�ػ��ϼ���+3��Ϊ+4����+5��Ϊ+4�����ȱ����ƽ��ԭ�������ӷ���ʽΪClO2��+ClO3��+2H+=2ClO2��+H2O
��ClO2����ɱ������ʱClԪ����+4��Ϊ-1����Cl2����ɱ������ʱClԪ����0��Ϊ-1����������ʵ���Cl2��ClO2ת�Ƶ��ӵ����ʵ���֮��Ϊ![]() ��ClO2�����������ǵ����ʵ���Cl2��
��ClO2�����������ǵ����ʵ���Cl2��![]() ����
����

����Ŀ�����������벻����ѧ�������Dz�������θҩ�����ű�ǩ�IJ�����Ϣ��
��������Ƭ Ӣ������Ferrous Sulfate Tablets ��Ʒ������������FeSO4��7H2O��ӦΪ��ʾ����95��0%~110��0% | ��������Ƭ Ӣ������Aluminium Hydroxide Tablets |
����Ҫ�ɷ֣����¡�̼��þ���������������� | |
����״����ƷΪ���£���ȥ���º��Ե���ɫ | ����Ӧ֢���ܻ���θ����� |
������ȡ��Ʒ����ȥ���£���ȡ������Լ�൱����������Ƭ0��2g������ϡ����1����ˮ20mL����ҡʹ���������ܽ⣬���ˣ���Һ�����������������εļ���Ӧ | ���÷����������ˣ��ڷ���һ��0��6~0��9g��һ��2~3Ƭ����һ��3�Σ���ǰ1Сʱ���� |
����ϸ�÷������˵���� |
I����������������Ƭ����ǩ�ش����⣺
��1����������Ƭ����ˮ����Һ����ɫ��_______________��
��2���������м���![]() �����ӷ�Ӧ����ʽ��______________________________________��
�����ӷ�Ӧ����ʽ��______________________________________��
��3�������У�����ϡ����1������������__________________________________________��
��4��������������Ƭ�Ƿ������ķ�����________________________________________��
II����������������Ƭ����ǩ�ش����⣺
��1���ܻ���θ�����ijɷ���__________________________________________��
��2��������������Ϊ����θ�������ڷ�ҩ����������������������______________��
A�� ���� B�� ���� C�� ���� D�� ������
��3��д�����������ĵ��뷽��ʽ��_____________________________________________��