ЬтФПФкШн
ЁОЬтФПЁПЯТСаа№ЪіжаВЛе§ШЗЕФЪЧ(вбжЊKsp(AgCl)ЃН4.0ЁС10Ѓ10ЃЌKsp(AgBr)ЃН4.9ЁС10Ѓ13ЃЌKsp(Ag2CrO4)ЃН2.0ЁС10Ѓ12)
A.0.lmolЁЄLЃ1 NH4HSШмвКжагаЃКc(NH4ЃЋ)ЃЋc(NH3ЁЄH2O)>c(HSЃ)ЃЋc(S2Ѓ)
B.НЋAgClКЭAgBrЕФБЅКЭШмвКЕШЬхЛ§ЛьКЯЃЌдйМгШызуСПЕФХЈAgNO3ШмвКЃЌAgClГСЕэжЪСПДѓгкAgBrГСЕэ
C.ЯђХЈЖШОљЮЊ1ЁС10Ѓ3mol/LЕФKClКЭK2CrO4ЛьКЯвКжаЕЮМг1ЁС10Ѓ3mol/LAgNO3ШмвКЃЌCrO42ЃЯШаЮГЩГСЕэ
D.ГЃЮТЯТЃЌpHЃН4.75ЁЂХЈЖШОљЮЊ0.l mol/LЕФCH3COOHЁЂCH3COONaЛьКЯШмвКЃКc(CH3COOЃ)ЃЋc(OHЃ)>c(CH3COOH)ЃЋc(HЃЋ)
ЁОД№АИЁПC
ЁОНтЮіЁП
A. ИљОнЮяСЯЪиКуЃЌ0.lmolЁЄLЃ1 NH4HSШмвКжагаЃКc(NH4ЃЋ)ЃЋc(NH3ЁЄH2O)=c(HSЃ)ЃЋc(S2Ѓ)+ c(H2S)ЃЌЫљвдc(NH4ЃЋ)ЃЋc(NH3ЁЄH2O)>c(HSЃ)ЃЋc(S2Ѓ)ЃЌЙЪAе§ШЗЃЛ
B. AgClЕФШмНтЖШДѓгкAgBrЃЌНЋAgClКЭAgBrЕФБЅКЭШмвКЕШЬхЛ§ЛьКЯЃЌc(ClЃ)ДѓЃЌдйМгШызуСПЕФХЈAgNO3ШмвКЃЌAgClГСЕэжЪСПДѓгкAgBrГСЕэЃЌЙЪBе§ШЗЃЛ
C. ЛьКЯШмвКЩњГЩТШЛЏвјГСЕэашвЊвјРызгЕФХЈЖШЪЧ![]() mol/LЃЌЩњГЩТШЛЏвјГСЕэашвЊвјРызгЕФХЈЖШЪЧ
mol/LЃЌЩњГЩТШЛЏвјГСЕэашвЊвјРызгЕФХЈЖШЪЧ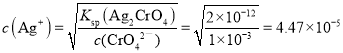 mol/LЃЌЫљвдClЃЯШГСЕэЃЌЙЪCДэЮѓЃЛ
mol/LЃЌЫљвдClЃЯШГСЕэЃЌЙЪCДэЮѓЃЛ
D. ГЃЮТЯТЃЌХЈЖШОљЮЊ0.l mol/LЕФCH3COOHЁЂCH3COONaЛьКЯШмвКЃЌpHЃН4.75ЃЌЫЕУїCH3COOЃЫЎНтаЁгкДзЫсЕчРыЃЌЫљвдc(CH3COOЃ)ЃОc(Na+)ЃОc(CH3COOH)ЃЌ ИљОнЕчКЩЪиКуЃЌc(CH3COOЃ)ЃЋc(OHЃ)= c(Na+)ЃЋc(HЃЋ)ЃЌЫљвдc(CH3COOЃ)ЃЋc(OHЃ)>c(CH3COOH)ЃЋc(HЃЋ)ЃЌЙЪDе§ШЗЁЃ

ЁОЬтФПЁПШэУЬПѓЕФжївЊГЩЗжMnO2ЃЌЛЙКЌгаFe2O3ЁЂAl2O3ЁЂSiO2ЕШЃЌгУШэУЬПѓНЌЮќЪеЙЄвЕЗЯЦјжаЕФЖўбѕЛЏСђЃЌжЦБИИпУЬЫсМиЕФСїГЬШчЭМЫљЪОЃК

вбжЊЃКТЫвКAжаЕФН№ЪєбєРызгжївЊЪЧMn2+ЛЙКЌгаЩйСПЕФFe2+ЁЂAl3+ЕШЃЌМИжжРызгПЊЪМГСЕэКЭЭъШЋГСЕэЪБЕФpHШчгвБэЫљЪОЃК
Рызг | ПЊЪМГСЕэЪБЕФpH | ЭъШЋГСЕэЪБЕФpH |
Fe2+ | 7.6 | 9.7 |
Fe3+ | 2.7 | 3.7 |
Al3+ | 3.8 | 4.7 |
Mn2+ | 8.3 | 9.8 |
ИљОнЩЯЪіСїГЬЃЌЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉаДГіШэУЬПѓЭЈШыЖўбѕЛЏСђЗЂЩњЕФжївЊЗДгІЕФЛЏбЇЗНГЬЪН__ЁЃ
ЃЈ2ЃЉТЫдќAЕФГЩЗжЪЧ__ЃЌЦфжаЕФЛЏбЇМќРраЭЪЧ___ЁЃ
ЃЈ3ЃЉВНжшЂкжаМгШыMnO2ЕФзїгУ__ЁЃ
ЃЈ4ЃЉгаЭЌбЇШЯЮЊПЩвдгУЬМЫсУЬЃЈMnCO3ЃЉЛђЧтбѕЛЏУЬ[Mn(OH)2]ЬцДњЪЏЛвШщЃЌФуЪЧЗёЭЌвтДЫЙлЕу___ЃПМђЪіРэгЩ___ЁЃ
ЃЈ5ЃЉТЫвКBжаМгШыKMnO4ЪБЗЂЩњЗДгІЕФРызгЗНГЬЪНЪЧ__ЁЃ
ЃЈ6ЃЉЕкЂпВНгУФјЦЌЃЈФјВЛВЮгыЗДгІЃЉКЭЬњАхзїЕчМЋЃЌЕчНтK2MnO4ШмвКПЩжЦБИKMnO4ЁЃгаЙиЫЕЗЈе§ШЗЕФЪЧ__ЃЈЬюзжФИЃЉ

A.aгыЕчдДЕФИКМЋЯрСЌ
B.РызгНЛЛЛФЄЮЊбєРызгНЛЛЛФЄ
C.бєМЋЕФЕчМЋЗДгІЮЊMnO42--e-=MnO4-
D.вѕМЋЕФЕчНтВњЮяЮЊKOH