��Ŀ����
����Ŀ��NA���������ӵ�������ֵ������˵����ȷ���ǣ� ��
A.��״���£�11.2LCl2����ˮ����Һ��Cl-��ClO-��HClO������֮��ΪNA
B.32.5gFeCl3ˮ���γɵ�Fe(OH)3����������Ϊ0.2NA
C.��״���£�4.48L������һ����̼�Ļ��������ȫȼ�գ����������ӵ���ĿΪ0.1NA
D.�ܱ�������2molNO��lmolO2��ַ�Ӧ������ķ�����Ϊ2NA
���𰸡�C
��������
A. Cl2����ˮ��������ˮ��Ӧ����Һ�к���Cl-��ClO-��HClO��Cl2�����������غ��֪Cl-��ClO-��HClO������֮�Ͳ���NA����A����
B. 32.5gFeCl3�����ʵ�����0.2mol���������������Ƕ�����ӵļ����壬����ˮ���γɵ�Fe(OH)3����������С��0.2NA����B����
C. ��״���£�4.48L������һ����̼�Ļ����������ʵ�����0.2mol������ȼ�յķ���ʽ![]() ��
��![]() ��������������һ����̼��������������ͬ������0.2mol�����������0.1mol��������C��ȷ��
��������������һ����̼��������������ͬ������0.2mol�����������0.1mol��������C��ȷ��
D. �ܱ�������2molNO��lmolO2��ַ�Ӧ����2molNO2������NO2����N2O4�����Բ���ķ�����С��2NA����D����

����Ŀ��������һ�ֳ��õĻ�ԭ����ijУ������ѧС��̽�����ᱻ�������������⡣
ʵ��� | �Լ� | ��Ϻ� ��ҺpH | ���� ��1h����Һ�� | ||
�Թ� | �ι� | ||||
| a | 4mL0.01mol��L1 KMnO4��Һ������ŨH2SO4 | 2mL0.3mol��L1H2C2O4 ��Һ | 2 | ��Ϊ��ɫ |
b | 4mL0.01mol��L1KMnO4��Һ������ŨNaOH | 7 | �����Ա仯 | ||
c | 4mL0.01mol��L1 K2Cr2O7��Һ������ŨH2SO4 | 2 | �����Ա仯 | ||
d | 4mL0.01mol��L1 K2Cr2O7��Һ������ŨNaOH | 7 | �����Ա仯 | ||
��1��H2C2O4�Ƕ�Ԫ���ᣬд��H2C2O4����ˮ�ĵ��뷽��ʽ��_____________��
��2��ʵ��I�Թ�a��KMnO4���ձ���ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ��________��
��3�������ͳ����������ϣ�ʵ��I�Թ�c��H2C2O4��K2Cr2O7��Һ��Ӧ������ʱ�������ɣ�������MnO2�ɴٽ�H2C2O4��K2Cr2O7�ķ�Ӧ�����ݴ����ϣ����������������ʵ��֤ʵ����һ�㡣
ʵ�� | ʵ��III | ʵ��IV | |
ʵ����� |
|
|
|
ʵ������ | 6 min�������ȫ�ܽ⣬��Һ��ɫ��dz���¶Ȳ��� | 6 min�����δ�ܽ⣬��Һ��ɫ�����Ա仯 | 6 min�����δ�ܽ⣬��Һ��ɫ�����Ա仯 |
ʵ��IV��Ŀ���ǣ�_______________________��
��4�������ȶ�ʵ��II��������̽����������Һ��Cr2O72- Ũ�ȱ仯��ͼ��
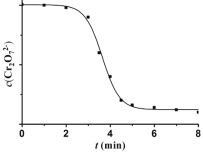
�갺Ͳ̲���Ϊ�˱仯��ͨ����������ʵ�ֵġ�
����i��MnO2��H2C2O4��Ӧ������Mn2+��
����ii��__________________________________��
�ٲ������ϣ���Һ��Mn2+�ܱ�PbO2����ΪMnO4-����Թ���i���ɲ������·���֤ʵ����0.0001molMnO2���뵽6mL____________�У�������ȫ�ܽ⣻����ȡ��������Һ���������PbO2���壬��ַ�Ӧ���ã��۲쵽_______________��
�ڲ����������ʵ�鷽��֤ʵ�˹���ii���������ǵ�ʵ�鷽����________��
��5���ۺ�����ʵ���֪�����ᷢ��������Ӧ��������__________________�йء�