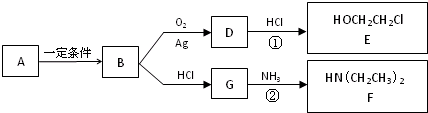
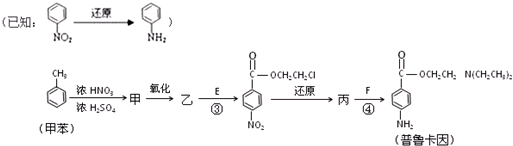
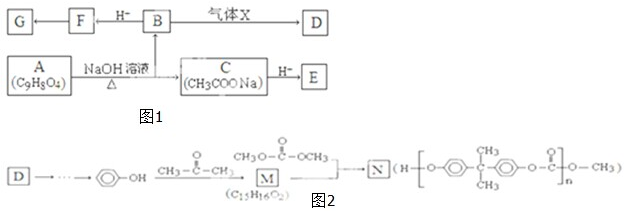
��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�-���ڱ��е�λ�ã��û�ѧ����ش��������⣺
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ ����Ԫ�ط��ţ���
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����[�ѧʽ] ��
��3���ܡ��ݡ�������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ�� ��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ�����Ľṹʽ�� ����������ݵ�ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ ��
��5��������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ ��
| �� ���� | IA | 0 | ||||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | �� | �� | �� | |||||
| 3 | �� | �� | �� | �� | ||||
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����[�ѧʽ]
��3���ܡ��ݡ�������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����ĵ���ʽ��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ�����Ľṹʽ��
��5��������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ
���㣺λ�ýṹ���ʵ����ϵӦ��,Ԫ�����ڱ��Ľṹ����Ӧ��,Ԫ�������ɺ�Ԫ�����ڱ����ۺ�Ӧ��
ר�⣺Ԫ����������Ԫ�����ڱ�ר��
������Ԫ�������ڱ��еķֲ�����֪����H������C������N������O������Na������Al������Si������Cl
��1�����Ӳ�Խ��뾶Խ���Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС��
��2��ͬһ����Ԫ�ص�ԭ�ӣ�����������������Ӧˮ�������������ǿ��
��3���ܡ��ݡ�������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����ΪNaClO��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ����ԭ�Ӹ�����Ϊ
��
=1��2��ΪCO2����ݵ�ͬ������������Ԫ�صĵ���Mg��Ӧ��������þ��C��
��5���ĵ���Al���Na������������ˮ����NaOH������Ӧ����ƫ�����ƺ���������ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaOH+3H2����
��1�����Ӳ�Խ��뾶Խ���Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС��
��2��ͬһ����Ԫ�ص�ԭ�ӣ�����������������Ӧˮ�������������ǿ��
��3���ܡ��ݡ�������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����ΪNaClO��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ����ԭ�Ӹ�����Ϊ
| 3 |
| 12 |
| 8 |
| 16 |
��5���ĵ���Al���Na������������ˮ����NaOH������Ӧ����ƫ�����ƺ���������ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaOH+3H2����
���
�⣺����Ԫ�������ڱ��еķֲ�����֪����H������C������N������O������Na������Al������Si������Cl��
��1�����Ӳ�Խ��뾶Խ������Na��Al��O�����Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС����Na��Al���ʴ�Ϊ��Na��Al��O��
��2���ڢ���C��N����ͬһ����Ԫ�ص�ԭ�ӣ�����Ԫ�������ɣ�����������������Ӧˮ�������������ǿ���������ԣ�HNO3��H2CO3���ڢ���C��Si����ͬ����Ԫ�أ�����Ԫ�������ɣ����ϵ�������������Ӧˮ�������������������ΪH2CO3��H2SiO3���ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3���ܡ��ݡ�������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����ΪNaClO�������ʽΪ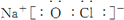 ���ʴ�Ϊ��
���ʴ�Ϊ��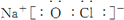 ��
��
��4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ����ԭ�Ӹ�����Ϊ
��
=1��2��ΪCO2���ṹʽΪO=C=O����ݵ�ͬ������������Ԫ�صĵ���Mg��Ӧ��������þ��C����ӦΪCO2+2Mg
2MgO+C���ʴ�Ϊ��O=C=O��CO2+2Mg
2MgO+C��
��5���ĵ���Al���Na������������ˮ����NaOH������Ӧ����ƫ�����ƺ���������ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaOH+3H2�������ӷ�ӦΪ2Al+2H2O+2OH-�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2H2O+2OH-�T2AlO2-+3H2����
��1�����Ӳ�Խ��뾶Խ������Na��Al��O�����Ӳ�һ�����ԭ�ӣ��˵����Խ��뾶ԽС����Na��Al���ʴ�Ϊ��Na��Al��O��
��2���ڢ���C��N����ͬһ����Ԫ�ص�ԭ�ӣ�����Ԫ�������ɣ�����������������Ӧˮ�������������ǿ���������ԣ�HNO3��H2CO3���ڢ���C��Si����ͬ����Ԫ�أ�����Ԫ�������ɣ����ϵ�������������Ӧˮ�������������������ΪH2CO3��H2SiO3���ʴ�Ϊ��HNO3��H2CO3��H2SiO3��
��3���ܡ��ݡ�������Ԫ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����ΪNaClO�������ʽΪ
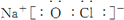 ���ʴ�Ϊ��
���ʴ�Ϊ��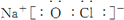 ��
����4���ɢں͢���ɣ��Ңںܵ͢�������Ϊ3��8�Ļ����ԭ�Ӹ�����Ϊ
| 3 |
| 12 |
| 8 |
| 16 |
| ||
| ||
��5���ĵ���Al���Na������������ˮ����NaOH������Ӧ����ƫ�����ƺ���������ѧ����ʽΪ��2Al+2NaOH+2H2O=2NaOH+3H2�������ӷ�ӦΪ2Al+2H2O+2OH-�T2AlO2-+3H2�����ʴ�Ϊ��2Al+2H2O+2OH-�T2AlO2-+3H2����
���������⿼��λ�á��ṹ���ʵ��ۺ�Ӧ�ã�Ϊ��Ƶ���㣬����Ԫ�������ڱ��е����ʿ��ƶϳ�Ԫ�ص����࣬���в����������ɵ�Ӧ�ã�ѧϰ��ע��������֪ʶ�����յ���ʽ����Ӧ����ʽ�����ӷ�Ӧ�Ȼ�ѧ�������д����Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��Ч���ܿ�ʱ��ҵϵ�д�
��Ч���ܿ�ʱ��ҵϵ�д�
�����Ŀ
��һ��һԪ��ROH����Һ�У�����һ��һԪ��HA��Һ������Ӧ����Һ�������ԣ������ж���һ����ȷ���ǣ�������
| A����Ӧ����Һ��C��A-��=C��R+�� |
| B����������������ʵ������ |
| C�����ɵ���RA���ܷ���ˮ�� |
| D�������һԪ��HA���� |
����˵����ȷ���ǣ�������
| A�������£������ʵ���Ũ�ȵ�������Һ�٣�NH4��2CO3��NH4Cl�ۣ�NH4��2Fe��SO4��2��c��NH4+�����٣��ڣ��� |
| B�������ʵ���Ũ�ȵ�H2S��NaHS�����Һ�У�c��Na+��+c��H+��=c��S2-��+c��HS-��+c��OH-�� |
| C�������£���c��H+��=1.0��10-13mol?L-1����Һ�У�Na+��S2-��AlO2-��SO32-�����Ӳ����ܴ������� |
| D��һ���¶��£�������μ����������Һ���������ԣ���ʱ��Һ�У�c��Na+����c��Cl-��=c��CH3COOH�� |
��ɫ����������Һ�У��ܴ���������ǣ�������
| A��Na+��K+��CO32-��NO3- |
| B��K+��Fe2+��NH4+��NO3- |
| C��NH4+��Al3+��SO42-��NO3- |
| D��K+��Na+��NO3-��OH- |
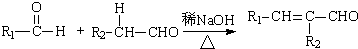 ��R��R��Ϊ�������⣩
��R��R��Ϊ�������⣩ ��R��R��Ϊ������
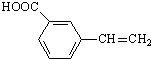
��R��R��������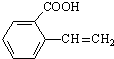 ��
�� ��
��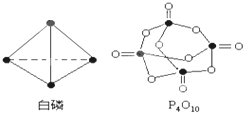 ���ף�P4�����������幹�͵ķ��ӣ��������������γ�P4O10ʱ��ÿ������ԭ��֮�����һ����ԭ�ӣ����⣬ÿ����ԭ������˫�����һ����ԭ�ӣ���ͼ��
���ף�P4�����������幹�͵ķ��ӣ��������������γ�P4O10ʱ��ÿ������ԭ��֮�����һ����ԭ�ӣ����⣬ÿ����ԭ������˫�����һ����ԭ�ӣ���ͼ��