��Ŀ����
����˵����ȷ���ǣ�������
| A�������£������ʵ���Ũ�ȵ�������Һ�٣�NH4��2CO3��NH4Cl�ۣ�NH4��2Fe��SO4��2��c��NH4+�����٣��ڣ��� |
| B�������ʵ���Ũ�ȵ�H2S��NaHS�����Һ�У�c��Na+��+c��H+��=c��S2-��+c��HS-��+c��OH-�� |
| C�������£���c��H+��=1.0��10-13mol?L-1����Һ�У�Na+��S2-��AlO2-��SO32-�����Ӳ����ܴ������� |
| D��һ���¶��£�������μ����������Һ���������ԣ���ʱ��Һ�У�c��Na+����c��Cl-��=c��CH3COOH�� |
���㣺����Ũ�ȴ�С�ıȽ�,���������ˮ��Һ�еĵ���ƽ��,����ˮ���Ӧ��,���ӹ�������
ר�⣺���ӷ�Ӧר��,����ƽ������Һ��pHר��,�����ˮ��ר��
������A�����Ӱ��NH4+ˮ��̶ȵ������жϣ�
B�����ݵ���غ��жϣ�
C��c��H+��=1.0��10-13mol?L-1����Һ�����ܳʼ��ԣ�Ҳ���ܳ����ԣ�
D���������ƣ���������Һ��ϣ�����֮�䷴Ӧ�����Ȼ��ƺʹ��ᣬ��Һ�����ԣ�������ƻ�ʣ�࣬���ݵ���غ�������غ��жϣ�
B�����ݵ���غ��жϣ�
C��c��H+��=1.0��10-13mol?L-1����Һ�����ܳʼ��ԣ�Ҳ���ܳ����ԣ�
D���������ƣ���������Һ��ϣ�����֮�䷴Ӧ�����Ȼ��ƺʹ��ᣬ��Һ�����ԣ�������ƻ�ʣ�࣬���ݵ���غ�������غ��жϣ�
���
�⣺A���������Ƚϣ�����笠����Ӻ�̼������ӷ�������ˮ�⣬������笠����Ӻ�������������ƣ���c��NH4+���ۣ��٣�����С����A����
B�������ʵ���Ũ�ȵ�H2S��NaHS�����Һ�д��ڵ���غ㣬Ϊc��Na+��+c��H+��=2c��S2-��+c��HS-��+c��OH-������B����
C��c��H+��=1.0��10-13mol?L-1����Һ�����ܳʼ��ԣ�Ҳ���ܳ����ԣ����������£�����֮�䲻�����κη�Ӧ���ɴ������棬��C����
D��ԭ��ֻ�д����Ƽ��������û�д��������Ҳû�������ӣ���n��Na+��=n��CH3COOH��+n��CH3COO-������ˣ���C��Na+��=C��CH3COOH��+C��CH3COO-������Ϊ�����ԣ�c��H+��=c��OH-���ӵ���غ��֪��C��Na+��+C��H+��=C��CH3COO-��+C��Cl-��+C��OH-�������Ǣ�C��Na+��=C��CH3COO-��+C��Cl-�������C��Na+����C��Cl-����Ȼ��Ѣٴ����ʽ����ȥC��Na+����c��Cl-��=c��CH3COOH������˵ó�C��Na+����C��Cl-��=c��CH3COOH������D��ȷ��
��ѡD��
B�������ʵ���Ũ�ȵ�H2S��NaHS�����Һ�д��ڵ���غ㣬Ϊc��Na+��+c��H+��=2c��S2-��+c��HS-��+c��OH-������B����
C��c��H+��=1.0��10-13mol?L-1����Һ�����ܳʼ��ԣ�Ҳ���ܳ����ԣ����������£�����֮�䲻�����κη�Ӧ���ɴ������棬��C����
D��ԭ��ֻ�д����Ƽ��������û�д��������Ҳû�������ӣ���n��Na+��=n��CH3COOH��+n��CH3COO-������ˣ���C��Na+��=C��CH3COOH��+C��CH3COO-������Ϊ�����ԣ�c��H+��=c��OH-���ӵ���غ��֪��C��Na+��+C��H+��=C��CH3COO-��+C��Cl-��+C��OH-�������Ǣ�C��Na+��=C��CH3COO-��+C��Cl-�������C��Na+����C��Cl-����Ȼ��Ѣٴ����ʽ����ȥC��Na+����c��Cl-��=c��CH3COOH������˵ó�C��Na+����C��Cl-��=c��CH3COOH������D��ȷ��
��ѡD��
���������⿼���Ϊ�ۺϣ��漰����ˮ�⡢����Ũ�ȴ�С�Ƚ��Լ����ӹ������⣬Ϊ�߿��������ͣ�������ѧ���ķ��������Ŀ��飬ע�����غ�����ã��Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�ѱ���Ϊ21���ͽ��������������ǡ����������ɴ����DZͧ����Ҫ���ϣ��о����֣�������ʯī������������������������CaF2-CaO������ʣ�������ͼ��ʾװ��ģ���ý����ƣ��趨�ڸ���ʯī���������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѣ�����������ȷ���ǣ�������
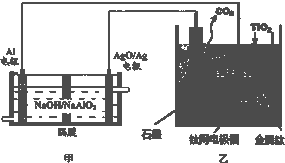

| A����װ�ù���������OH-��AgO/Ag���ƶ�����װ�ù���������O2-�������ƶ� | ||
| B����װ�������ĵ缫��ӦʽΪC+2O2--4e-�TCO2�� | ||
C������������Ч��Ϊ�ǣ����ȡ1mol Tiʱ����Al�����ʵ���
| ||
| D�����Ʊ�������ǰ������װ����CaO���������� |
����˵��������ǣ�������
| A����ѿ�����ڻ�ԭ�Ͷ��� |
B��һ���ܻ����ĽṹΪ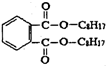 ��������������֬ ��������������֬ |
| C�������������Ի���������ᡢ�Ӧ������ |
| D���ý��ݹ����������Һ�Ĺ�������ˮ���ͷų�����ϩ���ɴﵽˮ�����ʵ�Ŀ�� |
���ж����л�����ڵ��칹�����Ŀ�жϴ�����ǣ�������
| A������ʽΪC4H9Cl���칹����ĿΪ4 |
| B������ʽΪC8H10���칹����ĿΪ4 |
| C������ʽΪC5H12O�Ĵ����칹����ĿΪ8 |
| D������ʽΪC6H12O2�������칹����ĿΪ20 |
�����������£����ܴ���������������ǣ�������
| A��c��H+��=1��10-14mol/L����Һ��K+��Cu2+��I-��SO42- |
| B��ˮ�������c��H+��=1��10-14mol/L����Һ��K+��Na+��AlO2-��S2O32- |
| C������Al��Ӧ����H2����Һ��NH4+��Ca2+��NO3-��I- |
| D������K3[Fe��CN��6]������ɫ��������Һ��H+��Na+��SO42-��CrO42- |
�� a+��b2+��c2-��d-���ֶ�����Ԫ�ص����ӣ������ʾ�����ͬ�ĵ��Ӳ�ṹ��������Ԫ�ص�ԭ��������ϵ�ǣ�������
| A��a��b��c��d |
| B��b��a��d��c |
| C��c��b��a��d |
| D��b��a��c��d |
 �������������������뷢չ����Ҫ���ʻ�������ʱ����������Ҫ��־��
�������������������뷢չ����Ҫ���ʻ�������ʱ����������Ҫ��־��