ЬтФПФкШн
ЁОЬтФПЁПвбжЊЃКЂйN2(g)+2O2(g)![]() 2NO2(g) ЁїH=+67.7kJ/mol
2NO2(g) ЁїH=+67.7kJ/mol
Ђк2N2H4(g)+2O2(g)![]() 2N2(g)+4H2O(g) ЁїH=-1068kJ/mol
2N2(g)+4H2O(g) ЁїH=-1068kJ/mol
ЃЈ1ЃЉЛ№М§ЗЂЩфЪБПЩгУыТЃЈN2H4ЃЉзїШМСЯЃЌвдЖўбѕЛЏЕЊзїбѕЛЏМСЃЌЫќУЧЯрЛЅЗДгІЩњГЩЕЊЦјКЭЫЎеєЦјЁЃИљОнИЧЫЙЖЈТЩаДГіN2H 4КЭNO2ЗДгІЕФШШЛЏбЇЗНГЬЪНЮЊ____ЃЛ
ЃЈ2ЃЉыТЃЈN2H4ЃЉКЭбѕЦјЗДгІЕФФмСПБфЛЏШчЭМЫљЪОЃЌЭМжаE1БэЪО___ЃЌE2БэЪО____ЃЌЁїH=-534kJ/molБэЪО_____ЁЃ

ЁОД№АИЁП2N2H4(g)+2NO2(g)![]() 3N2(g)+4H2O(g) ЁїH=-1135.7kJ/mol е§ЗДгІЕФЛюЛЏФм ФцЗДгІЕФЛюЛЏФм ыТЃЈN2H4ЃЉЕФШМЩеШШ
3N2(g)+4H2O(g) ЁїH=-1135.7kJ/mol е§ЗДгІЕФЛюЛЏФм ФцЗДгІЕФЛюЛЏФм ыТЃЈN2H4ЃЉЕФШМЩеШШ
ЁОНтЮіЁП
ЃЈ1ЃЉвбжЊЃКЂйЁЂN2(g)+2O2(g)![]() 2NO2(g) ЁїH=+67.7kJ/molЃЛ
2NO2(g) ЁїH=+67.7kJ/molЃЛ
Ђк2N2H4(g)+2O2(g)![]() 2N2(g)+4H2O(g) ЁїH=-1068kJ/molЃЛ
2N2(g)+4H2O(g) ЁїH=-1068kJ/molЃЛ
гЩИЧЫЙЖЈТЩЃЌЂк-ЂйЕУ2N2H4(g)+2NO2(g)![]() 3N2(g)+4H2O(g)ЃЌ
3N2(g)+4H2O(g)ЃЌ
ЙЪИУЗДгІЕФЁїH=-1068kJmol-1-67.7kJmol-1=-1135.7 kJmol-1ЃЌ
МД2N2H4(g)+2NO2(g)![]() 3N2(g)+4H2O(g) ЁїH=-1135.7kJ/molЃЛ
3N2(g)+4H2O(g) ЁїH=-1135.7kJ/molЃЛ
ЃЈ2ЃЉыТЃЈN2H4ЃЉКЭбѕЦјЗДгІЕФФмСПБфЛЏШчЭМЫљЪОЃЌЭМжаE1БэЪОе§ЗДгІЕФЛюЛЏФмЃЌE2БэЪОФцЗДгІЕФЛюЛЏФмЃЌЁїH=-534kJ/molБэЪОыТЃЈN2H4ЃЉЕФШМЩеШШЁЃ

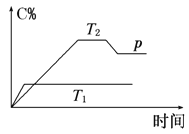
ЁОЬтФПЁПФГЪЕбщаЁзщЩшМЦгУ50mL1.0 mol/LбЮЫсИњ50mL1.1mol/LЧтбѕЛЏФЦШмвКдкШчЭМзАжУжаНјаажаКЭЗДгІЁЃдкДѓЩеБЕзВПЕцХнФЫмСЯЃЈЛђжНЬѕЃЉЃЌЪЙЗХШыЕФаЁЩеББПкгыДѓЩеББПкЯрЦНЁЃШЛКѓдйдкДѓЁЂаЁЩеБжЎМфЬюТњЫщХнФЫмСЯЃЈЛђжНЬѕЃЉЃЌДѓЩеБЩЯгУХнФЫмСЯАхЃЈЛђгВжНАхЃЉзїИЧАхЃЌдкАхжаМфПЊСНИіаЁПзЃЌе§КУЪЙЮТЖШМЦКЭЛЗаЮВЃСЇНСАшАєЭЈЙ§ЁЃЭЈЙ§ВтЖЈЗДгІЙ§ГЬжаЫљЗХГіЕФШШСППЩМЦЫужаКЭШШЁЃЪдЛиД№ЯТСаЮЪЬтЃК
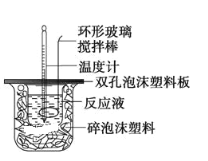
ЃЈ1ЃЉБОЪЕбщжагУЩдЙ§СПЕФNaOHЕФдвђНЬВФжаЫЕЪЧЮЊБЃжЄбЮЫсЭъШЋБЛжаКЭЁЃЪдЮЪЃКбЮЫсдкЗДгІжаШєвђЮЊгаЗХШШЯжЯѓЃЌЖјдьГЩЩйСПбЮЫсдкЗДгІжаЛгЗЂЃЌдђВтЕУЕФжаКЭШШ___ЃЈЬюЁАЦЋДѓЁБЁАЦЋаЁЁБЛђЁАВЛБфЁБЃЉЁЃ
ЃЈ2ЃЉИУЪЕбщаЁзщзіСЫШ§ДЮЪЕбщЃЌУПДЮШЁШмвКИї50 mLЃЌВЂМЧТМШчЯТдЪМЪ§ОнЁЃ
ЪЕбщађКХ | Ц№ЪМЮТЖШt1/Ёц | жежЙЮТЖШt2/Ёц | ЮТВю(t2-t1)/Ёц | ||
бЮЫс | NaOHШмвК | ЦНОљжЕ | |||
1 | 24.8 | 25.2 | 25.0 | 31.6 | 6.6 |
2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
3 | 25.2 | 25.0 | 25.1 | 31.9 | 6.8 |
вбжЊбЮЫсЁЂNaOHШмвКУмЖШНќЫЦЮЊ1.00g/cm3ЃЌжаКЭКѓЛьКЯвКЕФБШШШШнc=4.18ЁС10-3 kJ/(gЁЄЁц)ЃЌЛьКЯКѓШмвКжЪСПЮЊmЃЌЗДгІЗХГіЕФШШСПQ=cmЁїtЃЌдђИУЗДгІЩњГЩЫЎЕФЮяжЪЕФСПЮЊ____ЃЌжаКЭШШЮЊІЄH=____ЁЃ
ЃЈ3ЃЉШєгУЕШХЈЖШЕФбЮЫсгыNH3ЁЄH2OШмвКЗДгІЃЌдђВтЕУЕФжаКЭШШЛс___ЃЈЬюЁАЦЋДѓЁБЁАЦЋаЁЁБЛђЁАВЛБфЁБЃЉЃЌЦфдвђЪЧ____ЁЃ
ЃЈ4ЃЉдкжаКЭШШВтЖЈЪЕбщжаДцдкгУЫЎЯДЕгЮТЖШМЦЩЯЕФбЮЫсШмвКЕФВНжшЃЌШєЮоДЫВйзїВНжшЃЌдђВтЕУЕФжаКЭШШ____ЃЈЬюЁАЦЋДѓЁБЁАЦЋаЁЁБЛђЁАВЛБфЁБЃЉЁЃ