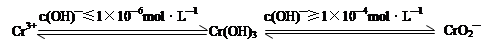��Ŀ����
I��NaOH��FeCl3����ѧ��ѧʵ���ҳ��õ��Լ���
��1����һ������������NaOH��Һ��Ӧ�Ĺ��嵥����________��________������������
����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����ʵ����ƫ�͵�ԭ����________��
| A������ƿ��ԭ����������ˮ |
| B��ϴ���ձ��Ͳ���������Һδת������ƿ�У� |
| C������ʱ�۲�Һ�温�� |
| D���ܽ��δ����ȴ����ת��������ƿ�� |
______________________________________________.
�������ȼ��ʱ�ܷ���������ȣ���Ҳ��Һ��ʯ��������Ҫ�ɷ֣���Ϊ��ԴӦ��������
���ճ����������
��֪��
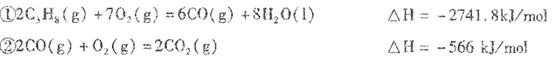
��1����Ӧ
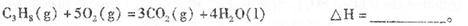
��2�����ݣ�1���еķ�Ӧ�������һ������ȼ�ϵ�أ�һ��ͨ�˿�������һ��ͨ��������壺ȼ�ϵ���ڲ������ڵIJ�����������(Y2O3)�������(ZrO2)���壬�����ڲ����Դ���O2�����ڵ���ڲ�O2������_________�������������������صĸ�����ӦΪ_____________________________.
��3��������ȼ�ϵ���ö��Ե缫�������Mg(NO3)2��NaCl�Ļ����Һ����ʼ������������Ϊ_______________________________________________��
��1�������裨�� B
��2��2 Fe3+ +Cu="=2" Fe2+ + Cu2+
3ClO-+2 Fe3++10OH-="2" FeO42-+3Cl-+5H2O
��1��-2219.2KJ/mol
��2���� C3H8+10O2-��20e-=3CO2+4H2O
��3���д�����ɫ���ݲ����������а�ɫ����������
�������������(l)��NaOH��Һ��Ӧ�Ĺ��嵥���н��������ͷǽ������ʹ���
����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ��A.����ƿ��ԭ����������ˮ,��Ӱ��������ҺŨ�ȡ�B.ϴ���ձ��Ͳ���������Һδת������ƿ�У����ʼ��٣�Ũ�ȼ�С��C. D�������������ܼ����������������ܼ���������ҺŨ��ƫ��
��2�� FeCl3���Ը�ʴ��·ͭ�壬����Fe3����ͭ���ʡ�
FeCl3 ��KClO��ǿ�����������Ʊ�K2FeO4������KClO��ǿ�����ԡ�
��1�����ø�˹���ɣ�����+�ڡ�3��/2, C3H8(g)+5O2(g)=3CO2(g)+4H2O (l) ��H=-2219.2KJ/mol
��2��ԭ��ص������Һ����������������صĸ�������������Ӧ��
��3�� �ö��Ե缫�������Mg(NO3)2��NaCl�Ļ����Һ������������ԭ��Ӧ��2H++2e-=H2��,������Χ��Һ��2OH-+Mg2+=" Mg" (OH)2��.

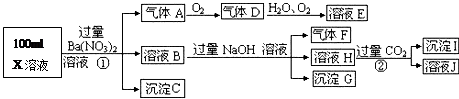
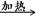 X��H2O��CO2��
X��H2O��CO2��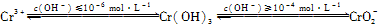
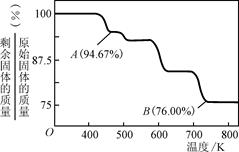
 8Cu��4FeO��2Fe2O3��16SO2
8Cu��4FeO��2Fe2O3��16SO2