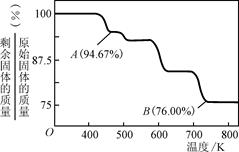
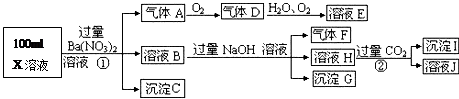
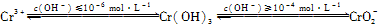��Ŀ����
��һƿ�������Һ�����п��ܺ�H+��NH4+��K+��Cu2+��Fe3+��CO32-��I-�е�һ�ֻ��֣�ȡ����Һ��������ʵ�飺
����pH��ֽ���飬������Һ��ǿ����
��ȡ������Һ�������������Ƶ���ˮ��������CCl4����CCl4������ɫ
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о���������
�ܽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵȷ�����ش�
��1������Һ�У��϶����ڵ��� ���϶������ڵ������� ��
��2������ȷ���Ƿ���ڵ�������_______��֤����(��)�Ƿ���ڵ�ʵ�鷽���� ��
��3��д��������漰�����ӷ���ʽ�����õ����ű������ת�Ƶķ������Ŀ ��
��4��д��������漰�����ӷ���ʽ ��
��10�֣�
��1��H+��I-��NH4+�� Cu2+��Fe3+��CO32-
��2��K+����ɫ��Ӧ
��3��Cl2+ 2I- = I2 + 2Cl-
��4��NH4+ + OH-  NH3��+H2O
NH3��+H2O
���������������1����pH��ֽ���飬������Һ��ǿ���ԣ���һ������H+��һ��������CO32-�������������Ƶ���ˮ��������CCl4����CCl4������ɫ��һ������I-����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������ת��Ϊ���ԣ��ڵμӹ����м��μ���Ϻ���Һ�о��������ɣ���һ��������Cu2+��Fe3+�����õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ��������һ������NH4+��
��2����ɫ��Ӧ��֤���Ƿ���K+��
��3��������ǿ������������������ǿ�Ļ�ԭ����������Ӧ�����ɵⵥ�ʣ�
��4��������ڼ��ȵ��������ǿ�ᷴӦ���ɰ�����
���㣺��������ʶ��

 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�ij��ɫ��Һ�������п��ܴ����������ӣ�Na+��Ag+��Ba2+��Al3+��AlO2-��S2-��CO32-��SO32-��SO42-����ȡ����Һ�����й����飬�������£�
�ش��������⣺
��1�����ɳ��������ӷ���ʽ�� ��
��֪�������������������ɣ�������ˮ������HBr�����������ʵ����һ���������ֳɷ֣��������Լ��Լ����������±��У��ɲ�����������ÿһ�ж�Ӧ��ȷ���ɵ÷֣�
| ���� | ʵ��Ŀ�� | �Լ� | ���� |
| 1 | | | |
| 2 | | | |
| 3 | | | |
| 4 | | | |
��2������Һ�����ɳ����ҵ����ӷ���ʽ�� ��
��3����������������Һ�п϶����ڵ������У� ��
��4�������Һ�п��ܴ��ڵ����ӣ���ʵ����֤���Ƿ���ڵķ�����
��