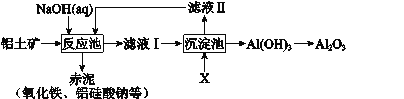
��Ŀ����
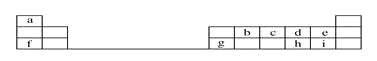
����Ŀ������һ����������������ˮ��Ӧ�IJ�ͬ��������������ʽ��з��࣬��ͼ��ʾ������գ�
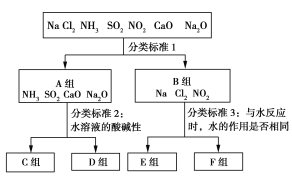
��1����ͼ�з����1���ֳ�A��B���������Ϊ______________________________��
��2����ҵ�ϳ���D����________���ѧʽ��������C�������ʶԴ�������Ⱦ���漰��Ӧ�Ļ�ѧ����ʽΪ______________________________ ��
��3��ʵ������Al3���Ʊ�Al��OH��3ʱ��Ӧѡ��D����________���ѧʽ����ˮ��Һ��
���ӷ���ʽΪ______________________________________��
��4��F�������������ʣ��ڹ�ҵ�Ͼ�����Ҫ����;�����д��һ����ѧ����ʽ��ʾ����;��
__________________________________��__________________________________________��
���𰸡���1���Ƿ���ˮ����������ԭ��Ӧ
��2��CaO��SO2��CaO===CaSO3��2CaSO3��O2===2CaSO4
��3��NH3��Al3����3NH3��H2O===Al��OH��3����3NH
��4��2Cl2��2Ca��OH��2===Ca��ClO��2��CaCl2��2H2O��3NO2��H2O===2HNO3��NO
��������
�����������1������ˮ�ֳ�AB��������ݣ�������Ӧ���̿�֪��NH3��SO2��CaO��Na2O����ˮ�������Ϸ�Ӧ���ɶ�Ӧ������Ƿ�������ԭ��Ӧ�� Na��Cl2��NO2����ˮ��Ӧ��������������ԭ��Ӧ�����������Ƿ���������ԭ��Ӧ��
�ʴ�Ϊ���Ƿ���������ԭ��Ӧ��
��2��A���е��������ˮ��Һ������Կ��Է�Ϊ����Һ�����ԣ�SO2����Һ�Լ��ԣ�NH3��CaO��Na2O���Կ�������Ⱦ����SO2����ͨ��ѡ��CaO������SO2�Կ�������Ⱦ����ӦΪ��CaO+SO2=CaSO3��2CaSO3��O2=2CaSO4��
�ʴ�Ϊ��CaO��CaO+SO2=CaSO3��2CaSO3��O2=2CaSO4��
��3��Al��OH��3������ǿ������ڰ�ˮ����ʵ������Al3+�Ʊ�Al��OH��3ʱ��Ӧѡ��D���е�NH3����ӦΪ��Al3++3NH3H2O=Al��OH��3+3NH4+��
�ʴ�Ϊ��NH3��Al3++3NH3H2O=Al��OH��3+3NH4+��
��4������ˮ�ķ�Ӧ�У�ˮ����������������NO2��ˮ�ķ�Ӧ�У�ˮ�Ȳ���������Ҳ������ԭ������F�������ΪCl2��NO2��Cl2����;����ȡƯ��Һ��Cl2+2NaOH=NaCl+NaClO+H2O��NO2��ҵ��ȡ���3NO2��H2O=2HNO3��NO��
�ʴ�Ϊ��Cl2+2NaOH=NaCl+NaClO+H2O��3NO2��H2O=2HNO3��NO��

 ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�
ȫ�ſ��䵥Ԫ�����������ܸ�ϰϵ�д�