��Ŀ����
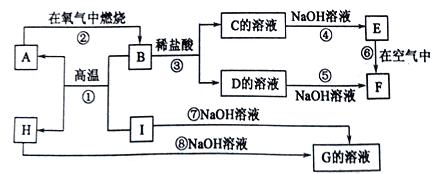
����Ŀ��A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮������ϵ��ͼ��ʾ(���ַ�Ӧ�������û���г�)����֪HΪ����������,F�Ǻ��ɫ������ˮ�ij�������A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
����д���пհ���
(1)A��B��C��D��E��F����������������ͬһ��Ԫ�ص�������____________��
(2)д��C��H�Ļ�ѧʽ��C___��H______��
(3)д����Ӧ�١����Ļ�ѧ����ʽ����Ӧ��_______________����Ӧ��________________��
(4)��Ӧ�������г��ֵ�������_______________��
���𰸡� �� FeCl2 Al2O3 8Al + 3Fe3O4![]() 4Al2O3+9Fe 2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2�� ��ɫ�����ڿ�����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ
4Al2O3+9Fe 2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2�� ��ɫ�����ڿ�����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ
��������A��I�ֱ��ʾ��ѧ��ѧ�г�����һ�����ʣ�����֮������ϵ��ͼ��ʾ(���ַ�Ӧ�������û���г�)����֪HΪ���������H����������������������Һ��Ӧ����G��ƫ�����ơ�I������������Һ��ӦҲ����G��B��I�ڸ����·�Ӧ����A��H����÷�Ӧ�����ȷ�Ӧ��I������A��������ȼ������B��F�Ǻ��ɫ������ˮ�ij�����F����������������A������B��������������D���Ȼ�����C���Ȼ�������E���������������ݴ˽��
�������Ϸ�����֪A��Fe��B��Fe3O4��C��FeCl2��D��FeCl3��E��Fe(OH)2��F��Fe(OH)3��G��Na[Al(OH)4]��H��Al2O3��I��Al����
(1)�������Ϸ�����֪A��B��C��D��E��F����������������ͬһ��Ԫ�ص�����������
(2)C��H�Ļ�ѧʽ�ֱ���FeCl2 ��Al2O3��
(3)��Ӧ�������ȷ�Ӧ������ʽΪ8Al+3Fe3O4![]() 4Al2O3+9Fe����Ӧ������������������Һ��Ӧ����ѧ����ʽΪ2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2����
4Al2O3+9Fe����Ӧ������������������Һ��Ӧ����ѧ����ʽΪ2Al+2NaOH+6H2O=2Na[Al(OH)4]+3H2����
(4)���������������ױ�����Ϊ������������Ӧ�����г��ֵ������ǰ�ɫ�����ڿ�����Ѹ�ٱ�ɻ���ɫ������ɺ��ɫ��

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�����Ŀ���¶�ΪT1ʱ���������ݻ���Ϊ1L�ĺ����ܱ������н�������Ӧ: 2NO2(g)![]() 2NO(g)+O2(g) (����Ӧ����)��ʵ����:v��=v(NO2)����=k��c2(NO2)��v��=v(NO)����= 2v(O2)����=k��c2(NO)��c(O2)��k����k��Ϊ���ʳ��������¶�Ӱ�졣
2NO(g)+O2(g) (����Ӧ����)��ʵ����:v��=v(NO2)����=k��c2(NO2)��v��=v(NO)����= 2v(O2)����=k��c2(NO)��c(O2)��k����k��Ϊ���ʳ��������¶�Ӱ�졣
����˵����ȷ����
���� ��� | ���ʵ���ʼŨ�ȣ�mol��L��1�� | ���ʵ�ƽ��Ũ�ȣ�mol��L��1�� | ||
c(NO2) | c(NO) | c(O2) | c(O2) | |
�� | 0.6 | 0 | 0 | 0.2 |
�� | 0.3 | 0.5 | 0.2 | |
�� | 0 | 0.5 | 0.35 | |
A. ��kΪ�÷�Ӧ�Ļ�ѧƽ�ⳣ��������k=k��:k��
B. ��ƽ��ʱ�����������������е���ѹǿ֮��Ϊ20��17
C. ����������ʼƽ�������ƶ�����ƽ��ʱ����������NO2��ת���ʱ��������е�С
D. ���ı��¶�ΪT2,��T2>T1,��k��:k��<0.8
����Ŀ��I.��A��B��C��D���ֽ������±���װ�ý���ʵ�顣
װ�� |
|
|
|
���� | ���۽���A�����ܽ� | C���������� | A����������� |
����ʵ������ش��������⣺
(1)װ�ü��и����ĵ缫��Ӧʽ��______________________________________��
(2)װ�����������ĵ缫��Ӧʽ��________________________________________��
(3)���ֽ�����������ǿ������˳����______________________��
II���ֱ�ͼ��ʾ�ס���װ�ý���ʵ�飬ͼ�������ձ��е���ҺΪ��ͬŨ�ȵ�ϡ���ᣬ����AΪ��������
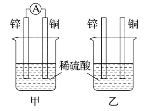
(1)����������ȷ����________��
A�����ձ���ͭƬ����������ݲ��� B������ͭƬ������������ͭƬ������
C�����ձ�����Һ�е�H��Ũ�Ⱦ���С D���������ݵ����ʼ��еı����е���
(2)��װ���У�ijͬѧ���ֲ���ͭƬ�������ݲ�����пƬ��Ҳ���������壬ԭ�������________________________________________________________________________��
(3)��װ���У�����ϡ���ỻ��CuSO4��Һ����д��ͭ�缫�ĵ缫��Ӧ________________________��