��Ŀ����
����Ŀ����ν��ʹ�����CO2�ĺ�������Ч�ؿ�������CO2�ǵ�ǰ��ѧ���о�����Ҫ���⡣
(1)��ѧ����H2��CO2�����״�ȼ�ϡ�Ϊ̽���÷�Ӧԭ������������ʵ�飺ij�¶��£����ݻ�Ϊ2 L���ܱ������г���1 mol CO2��3.25 mol H2�����¶�һ���������·�Ӧ�����CO2��CH3OH(g)��H2O(g)�����ʵ���(n)��ʱ��ı仯��ϵ��ͼ��ʾ��
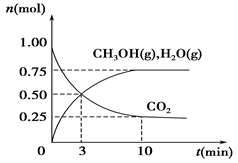
�ٴӷ�Ӧ��ʼ��3 minʱ��������ƽ����Ӧ����v(H2)��________��
�����д�ʩ��һ����ʹCO2��ת�����������________��
A����ԭ�������ٳ���1 mol CO2 B����ԭ�������ٳ���1 mol H2
C����ԭ�����г���1 mol He D��ʹ�ø���Ч�Ĵ���
E����С�������ݻ� F����ˮ��������ϵ�з����
����˵����Ӧ�Ѵ�ƽ��״̬����________(����ĸ,��ͬ)��
A.v(H2)=3v(CO2) B.������ѹǿ���ֲ���
C.v��(CO2)=v��(CH3OH) D.�������ܶȱ��ֲ���
(2)��ѧ�һ�����������һ���������������̼��Ӧ�����Ҵ�ȼ�ϣ����Ȼ�ѧ��Ӧ����ʽΪ2CO2(g)��6H2(g) ![]() CH3CH2OH(g)��3H2O(g)����H��a kJ��mol��1����һ��ѹǿ�£���ø÷�Ӧ��ʵ�����������ʾ������ݱ������ݻش��������⡣
CH3CH2OH(g)��3H2O(g)����H��a kJ��mol��1����һ��ѹǿ�£���ø÷�Ӧ��ʵ�����������ʾ������ݱ������ݻش��������⡣
n(H2)/n(CO2) | 500 | 600 | 700 | 800 |
1.5 | 45 | 33 | 20 | 12 |
2 | 60 | 43 | 28 | 15 |
3 | 83 | 62 | 37 | 22 |
��������Ӧ��a________0(����ڡ���С�ڡ�)��
�ں����£���Ӧ��ϵ�м�������������Ӧ����������________(�������С�����䡱)��
������![]() ��ֵ���������Ҵ������ʵ���________(�������С�������䡱����ȷ����)��
��ֵ���������Ҵ������ʵ���________(�������С�������䡱����ȷ����)��
���𰸡� 0.25 mol��L��1��min��1 B E F B C С�� ���� ����ȷ��
��������(1) ���ӷ�Ӧ��ʼ��3minʱ��V(CO2)=(1-0.5)/2��3=1/12 mol��L��1��min��1 �����ݷ�Ӧ����ʽ��CO2+3H2 ![]() CH3OH(g)+H2O(g)����ѧ������֮�ȵ��ڻ�ѧ��Ӧ����֮�ȣ���V(H2)=0.25 mol��L��1��min��1����ȷ�𰸣�0.25 mol��L��1��min��1��
CH3OH(g)+H2O(g)����ѧ������֮�ȵ��ڻ�ѧ��Ӧ����֮�ȣ���V(H2)=0.25 mol��L��1��min��1����ȷ�𰸣�0.25 mol��L��1��min��1��
����ԭ�������ٳ���1molCO2��CO2�������ʵ���������,��Ȼƽ���������ƶ�,������������ԭ������֪��, CO2��ת���ʼ�С��A������ԭ�������ٳ���1molH2��ƽ��������CO2��ת��������B��ȷ����ԭ�����г���1molHe����ƽ��û��Ӱ�죬CO2��ת���ʲ��䣬C����ʹ�ø���Ч�Ĵ�����ֻ�ܼӿ췴Ӧ���ʣ�ƽ�ⲻ�ƶ���CO2��ת���ʲ��䣬D������С�������ݻ����൱�ڼ�ѹ��ƽ�����ƣ�CO2��ת��������E��ȷ����ˮ��������ϵ�з��������С��ˮ������Ũ�ȣ�ƽ��������CO2��ת��������F��ȷ����ȷ�𰸣�B E F��
�ۻ�ѧ������֮�ȵ��ڻ�ѧ��Ӧ����֮����v(H2)=3v(CO2)����û��ָ�����淴Ӧ�������ж���Ӧ�Ѵ�ƽ��״̬��A���÷�Ӧ��һ����Ӧǰ�����������Ļ�ѧ��Ӧ���÷�Ӧ�ﵽƽ��״̬������������ѹǿ���䣬B��ȷ����ѧ������֮�ȵ��ڻ�ѧ��Ӧ����֮�����ұ������淴Ӧ�������ж���Ӧ�Ѵ�ƽ��״̬��C��ȷ����Ӧǰ����������������䣬������������䣬���۸÷�Ӧ�Ƿ�ﵽƽ��״̬�������������ܶ�ʼ�ղ��䣬D������ȷѡ��B C��
(2)����ͼ�������жϣ����¶����ߣ�������̼ת���ʼ�С��˵��ƽ��������У����������ȷ�Ӧ�������Ƿ��ȷ�Ӧ��aС��0����ȷ�𰸣�С�ڡ�
�������Ի�ѧƽ��û��Ӱ��,���Լ������,ƽ�ⲻ�ƶ�,��Ӧ��Ӧ�Ȳ��䣬��ȷ�𰸣����䡣
(3)�����Ͷ�����̼���ʵ�����ֵ����,��������������,Ҳ����Ϊ��С������̼,���Զ�����̼ת�����������ɵ��Ҵ������ʵ�����һ��������С����ȷ�𰸣�����ȷ����