��Ŀ����
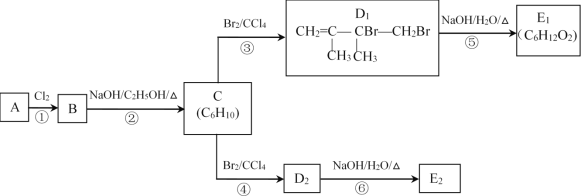
����Ŀ��������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1-�嶡��ķ�Ӧ���£�
NaBr+H2SO4=HBr+NaHSO4 ��
R-OH+HBr![]() R-Br+H2O ��
R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȡ��й������б����£�
�Ҵ� | ������ | ������ | 1-�嶡�� | |
�ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
�е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��ش�
��1���õ����������к��������Ҵ���Ϊ���Ƶô����������飬��������ˮϴ�ӣ���Һ���ټ�����ˮCaCl2��Ȼ����е�ʵ�������___������ĸ����
a����Һ b������ c����ȡ d������
��2���������ˮ����___�Ҵ���������������������������С����������ԭ����___��
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�������___�������ϲ��������²����������ֲ�������
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����___������ĸ����
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��5��Ϊ�˼����������к�����Ԫ�أ�ͨ�����õķ����ǣ�ȡ���������飬Ȼ��������в������ټ��� �ڼ���AgNO3��Һ �ۼ���ϡHNO3�ữ �ܼ���NaOH��Һ�����в���˳���������___������ĸ����
a���٢ڢۢ� b���ڢۢ٢� c���ܢ٢ۢ� d�� �٢ܢڢ�
��6�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������������___��
���𰸡�b С�� �Ҵ����ӿ���ˮ�����γ��������������Ӳ�����ˮ�����γ���� �²� abc c ƽ��������������ķ����ƶ�����Ӧ�������ƶ���
��������
��ʵ����NaBr+H2SO4�����������봼��Ӧ�������Ũ�ȣ���Ҫ�跨����HBr�Ļӷ���ʧ���ӱ������ݿ�֪���������1-�嶡���ܶȱ�ˮ�Ĵ�����������±������������ˮ��������ķе��������ֵ͵ö�������ͨ������������з��롣
��1���������к��������Ҵ���������ˮϴ�ӣ���Һ�ɳ�ȥ���Ҵ����ټ�����ˮCaCl2���������е�ˮ��ʣ����Ҵ��������ܽ�����CaCl2��Ϊ���ܺͲ��ܵ�CaCl2�����������ʷ��룬֮���ʵ�����ӦΪ����Ϊ��b
��2���Ҵ����ں�-OH������ˮ�����γ�������Ӷ���ˮ���ܣ����������C-H��C-Br�����Բ���ǿ����������ˮ�γ���������������ˮ�������������ˮ����С���Ҵ�����Ϊ��С�ڣ��Ҵ����ӿ���ˮ�����γ��������������Ӳ�����ˮ�����γ����
��3�����������Ϣ��1-�嶡����ܶ�Ϊ1.2758gcm-3����ˮ�Ĵ����Խ�1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã��������������²㡣��Ϊ���²�
��4�����������Ϣ��Ũ������������ȡ��Ӧ����HBr����Ũ��ʹ���ɵ�HBr�����ӷ�����ʧ��ͬʱ������Ũ����Ĵ�������ˮ����ϩ���ѡ�Br-��Ũ��������ΪBr2�������Ʊ������У������Ũ����������ϡ�͡���Ϊ��abc
��5���������е��岻����AgNO3��Һ��Ӧ��Ӧʹ�������е���ת���Br-�ټ��飬�ʼ����������к��е���Ԫ�ؿɲ��õķ����ǣ�ȡ���������飬����NaOH��Һ�����ȣ���Ӧ����ȴ������ϡHNO3�ữ���ټ���AgNO3��Һ�����Բ���˳��Ϊ���ܢ٢ۢڡ���Ϊ��c
��6��������������ԭ�������Ͳ����Ũ�ȣ�ƽ��������Ӧ�����ƶ�����������߲��ʡ��������Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������������ƽ��������������ķ����ƶ�����Ӧ�������ƶ�������Ϊ��ƽ��������������ķ����ƶ�����Ӧ�������ƶ���