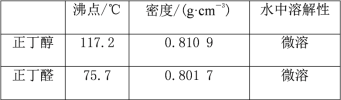��Ŀ����
����Ŀ��ij�������A��һ�������¿ɷ�����ͼת����
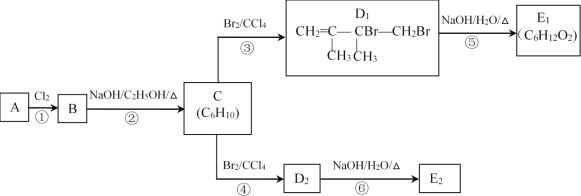
��֪��A������ͼ��������Է�������Ϊ84����˴Ź�������ֻ��һ���⡣E1��E2��Ϊͬ���칹�塣
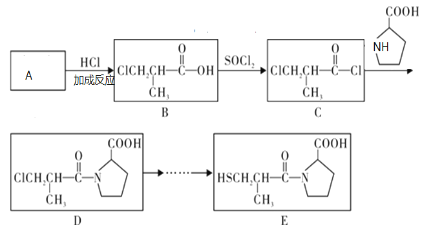
��1��D1�Ƿ����˳���칹___����������������������
��2�������ķ�Ӧ�У����ڼӳɷ�Ӧ����___��
��3��C�Ļ�ѧ������___��
��4��д��A��һ�������·����Ӿ۷�Ӧ�Ļ�ѧ����ʽ___��
��5��E2�Ľṹ��ʽΪ___��
��6��д����Ӧ�ڵĻ�ѧ����ʽ___��
���𰸡��� �٢ۢ� 2��3-����-1��3-����ϩ 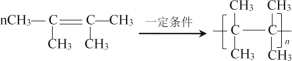
![]()
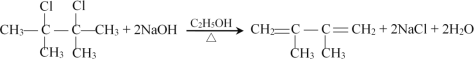
��������
��A���Ƶ���![]() =6�����ƶϷ���ʽΪC6H12����������˴Ź�������ֻ��һ���⣬��A��������Ӧ�IJ����ܷ�����ȥ��Ӧ����C6H10����AΪ
=6�����ƶϷ���ʽΪC6H12����������˴Ź�������ֻ��һ���⣬��A��������Ӧ�IJ����ܷ�����ȥ��Ӧ����C6H10����AΪ![]() ����������ʾת������Ϊ��
����������ʾת������Ϊ��![]() �����������ӳɷ�Ӧ����B:
�����������ӳɷ�Ӧ����B: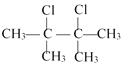 ������D1�Ľṹ��֪��B��ȥ��C��
������D1�Ľṹ��֪��B��ȥ��C��![]() ��C����Ӧ����Br21:1�����ӳɷ�Ӧ����D1��
��C����Ӧ����Br21:1�����ӳɷ�Ӧ����D1��![]() ���پ���Ӧ��ˮ��ΪE1��
���پ���Ӧ��ˮ��ΪE1��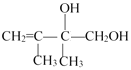 ������E1��E2��Ϊͬ���칹�壬���ƶϣ�C����Ӧ����Br21:1�����ӳɷ�Ӧ����D2��
������E1��E2��Ϊͬ���칹�壬���ƶϣ�C����Ӧ����Br21:1�����ӳɷ�Ӧ����D2��![]() ����ˮ��ΪE2��
����ˮ��ΪE2��![]() �����������Ƶ����������л�֪ʶ���ɽ��С�⡣
�����������Ƶ����������л�֪ʶ���ɽ��С�⡣
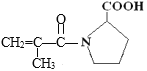
��1�����ݷ�����֪��D1Ϊ![]() ��1��̼��2��ԭ����ͬ����ΪH����������˳���칹����Ϊ����
��1��̼��2��ԭ����ͬ����ΪH����������˳���칹����Ϊ����
��2����Ӧ��Ϊ![]() �������ӳɣ���Ӧ��Ϊ
�������ӳɣ���Ӧ��Ϊ ����ȥ��Ӧ����Ӧ�ۡ���Ϊ
����ȥ��Ӧ����Ӧ�ۡ���Ϊ![]() ��Br2�ļӳɷ�Ӧ����Ӧ�ݡ���Ϊ±������ˮ�ⷴӦ������ȡ����Ӧ�����Ԣ����������ڼӳɷ�Ӧ���Ǣ٢ۢܡ���Ϊ���٢ۢܣ�
��Br2�ļӳɷ�Ӧ����Ӧ�ݡ���Ϊ±������ˮ�ⷴӦ������ȡ����Ӧ�����Ԣ����������ڼӳɷ�Ӧ���Ǣ٢ۢܡ���Ϊ���٢ۢܣ�
��3��C���ڶ�ϩ��������ϵͳ��������C�Ļ�ѧ������2��3-����-1��3-����ϩ����Ϊ��2��3-����-1��3-����ϩ��
��4��A��̼̼˫������һ���������ܷ����Ӿ۷�Ӧ����ѧ����ʽΪ��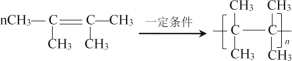 ����Ϊ��
������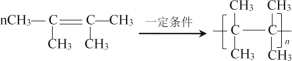
��5��D2��![]() ���ڼ���������ˮ��õ���
���ڼ���������ˮ��õ���![]()
![]() ������
������![]() ��
��
��6����Ӧ���� ��NaOH�Ĵ���Һ�м��ȷ�������ȥ��Ӧ����ѧ����ʽΪ��
��NaOH�Ĵ���Һ�м��ȷ�������ȥ��Ӧ����ѧ����ʽΪ��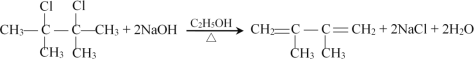 ����Ϊ��
������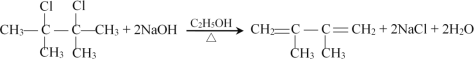 ��
��

 Сѧ��ʱ��ҵȫͨ����ϵ�д�
Сѧ��ʱ��ҵȫͨ����ϵ�д� �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
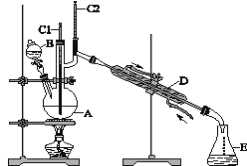
�»ƸԱ����ܾ�ϵ�д�����Ŀ��������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1-�嶡��ķ�Ӧ���£�
NaBr+H2SO4=HBr+NaHSO4 ��
R-OH+HBr![]() R-Br+H2O ��
R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȡ��й������б����£�
�Ҵ� | ������ | ������ | 1-�嶡�� | |
�ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
�е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��ش�
��1���õ����������к��������Ҵ���Ϊ���Ƶô����������飬��������ˮϴ�ӣ���Һ���ټ�����ˮCaCl2��Ȼ����е�ʵ�������___������ĸ����
a����Һ b������ c����ȡ d������
��2���������ˮ����___�Ҵ���������������������������С����������ԭ����___��
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�������___�������ϲ��������²����������ֲ�������
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����___������ĸ����
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
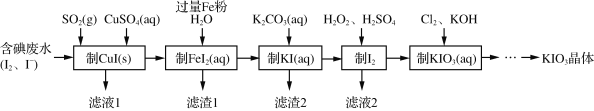
��5��Ϊ�˼����������к�����Ԫ�أ�ͨ�����õķ����ǣ�ȡ���������飬Ȼ��������в������ټ��� �ڼ���AgNO3��Һ �ۼ���ϡHNO3�ữ �ܼ���NaOH��Һ�����в���˳���������___������ĸ����
a���٢ڢۢ� b���ڢۢ٢� c���ܢ٢ۢ� d�� �٢ܢڢ�
��6�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������������___��