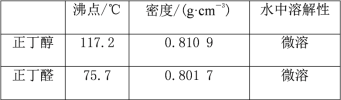��Ŀ����
����Ŀ�������£�ȡ10 mL 0.1 molL1ij��Ԫ��H2A���μ�0.1 molL1 NaOH��Һ����֪��H2A��H+ + HA����HA��![]() H+ + A2��������˵����ȷ����
H+ + A2��������˵����ȷ����
A.A2���ɾ�������ˮ��õ�H2A
B.����ȥNaOH��Һ���10 mLʱ����Һ������Ũ�ȴ�С˳��Ϊ��c(Na+)>c(HA��)>c(H+)>c(A2��)>c(OH��)
C.���μ�������ʱ����Һ����c(Na+)��c(HA��) + c(A2��)
D.����ȥNaOH��Һ���20 mLʱ����ʱ��Һ����c(Na+)��c(HA��) + c(A2��)
���𰸡�B
��������
A. H2AΪǿ����ʣ���ȫ��������H+ ��HA����A2���ɾ���ˮ��ֻ�ܵõ�HA-��A����
B. ����ȥNaOH��Һ���10 mLʱ����Һ�е�����ΪNaHA������Ũ�ȴ�С˳��Ϊ��c(Na+)>c(HA��)>c(H+)>c(A2��)>c(OH��)��B��ȷ��
C. ���μ�������ʱ��������Һ�ʵ����ԣ�c(Na+) +c(H+)=c(HA��)+2c(A2��)+c(OH��)��c(H+)= c(OH��)����Һ����c(Na+)��c(HA��) + 2c(A2��)��C����
D. ����ȥNaOH��Һ���20 mLʱ������ΪNa2A�����������غ��ʱ��Һ����c(Na+)��2c(HA��) + 2c(A2��)��D����
��ΪB��

 ���Ͱ�ͨ��ĩ���ϵ�д�
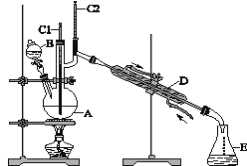
���Ͱ�ͨ��ĩ���ϵ�д�����Ŀ��������±�ᷴӦ���Ʊ�±��������Ҫ������ʵ�����Ʊ��������1-�嶡��ķ�Ӧ���£�
NaBr+H2SO4=HBr+NaHSO4 ��
R-OH+HBr![]() R-Br+H2O ��
R-Br+H2O ��
���ܴ��ڵĸ���Ӧ�У�����Ũ����Ĵ�������ˮ����ϩ���ѣ�Br-��Ũ��������ΪBr2�ȡ��й������б����£�
�Ҵ� | ������ | ������ | 1-�嶡�� | |
�ܶ�/g��cm-3 | 0.7893 | 1.4604 | 0.8098 | 1.2758 |
�е�/�� | 78.5 | 38.4 | 117.2 | 101.6 |
��ش�
��1���õ����������к��������Ҵ���Ϊ���Ƶô����������飬��������ˮϴ�ӣ���Һ���ټ�����ˮCaCl2��Ȼ����е�ʵ�������___������ĸ����
a����Һ b������ c����ȡ d������
��2���������ˮ����___�Ҵ���������������������������С����������ԭ����___��
��3����1-�嶡��ֲ�Ʒ���ڷ�Һ©���м�ˮ�����ã�������___�������ϲ��������²����������ֲ�������
��4���Ʊ������У������Ũ����������ϡ�ͣ���Ŀ����___������ĸ����
a�����ٸ�����ϩ���ѵ����� b������Br2������
c������HBr�Ļӷ� d��ˮ�Ƿ�Ӧ�Ĵ���
��5��Ϊ�˼����������к�����Ԫ�أ�ͨ�����õķ����ǣ�ȡ���������飬Ȼ��������в������ټ��� �ڼ���AgNO3��Һ �ۼ���ϡHNO3�ữ �ܼ���NaOH��Һ�����в���˳���������___������ĸ����
a���٢ڢۢ� b���ڢۢ٢� c���ܢ٢ۢ� d�� �٢ܢڢ�
��6�����Ʊ�������ʱ�����ñ߷�Ӧ����������ķ�������������___��