��Ŀ����
����Ŀ�����������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʣ�������������ȡ��������������ͼ��ʾ��
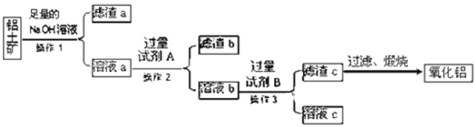
��1������1��������______������Ҫ����Ҫ���������У�______��
��2���Լ�A��______�����û�ѧʽ�ش𣩣�
��3����Һb���Լ�B��Ӧ�����ӷ���ʽΪ______________________��
��4����д���������������NaOH��Һ���������йط�Ӧ��ѧ����ʽ��______________��
��5��ijͬѧ��Ϊ��������Һa��ͨ����ǹ�����CO2��Ȼ��ֱ�ӽ��õ�������b���պ�Ҳ�ɵõ�Al2O3�����ҿ��Լ�������ȡ�����������̣�����Ϊ��ͬѧ�Ŀ���������______������������������������ǣ�______��������Ϊ������������Բ��ش�
��6��������ڵ����������Ʊ���������2Al2O3![]() 4Al+3O2�������ڱ�״��������2.24L����������÷�Ӧת�Ƶĵ�����Ϊ______��
4Al+3O2�������ڱ�״��������2.24L����������÷�Ӧת�Ƶĵ�����Ϊ______��
���𰸡����� ���������ձ���©�� HCl(��HNO3) H++NH3H2O=NH4++H2O ��Al3++3NH3��H2O =Al(OH)3��+3NH4+ Al2O3+2NaOH�T2NaAlO2+H2O��SiO2+2NaOH�TNa2SiO3+H2O ������ Al2O3�к���SiO2���� 0.4NA
��������
SiO2���������������������Ʒ�Ӧ���ܽ⣬����������м����������ƣ��õ���Һa�к���ƫ��������ӡ���������ӣ�����aΪ��������a��Һ�������A��Һ����ȥ��������ӣ�AΪ����Һ����ƫ���������ת��Ϊ�����ӣ�������Һb�У��ӹ���B������������������BΪ��ˮ�������������ȷֽ�����������ݴ˷������
(1)����1Ϊ���벻�������Һ�IJ�����Ϊ���ˣ�������������Ϊ������̨��©�������������ձ����ʴ�Ϊ�����ˣ���������©�����ձ���
����aΪ���������ʲ��ù��˵ķ������룬�ʴ�Ϊ�����ˣ���������©�����ձ���
(2)����������������ƣ��õ���Һa�к���ƫ��������ӡ���������ӣ��������A��Һ����ȥ��������ӣ���AΪ����������������Һ���ʴ�Ϊ��HCl��
(3)��Һb�к��������ӡ����������ᣬҪʹ�����ӳ���������������ˮ����ˮ�����ᷴӦ�����Ȼ�泥���ˮ���Ȼ�����Ӧ�����Ȼ�狀�����������������Ӧ�ķ���ʽΪHCl+NH3H2O=NH4Cl+H2O��AlCl3+3NH3H2O=Al(OH)3��+3NH4Cl�����ӷ���ʽΪ��H++NH3H2O=NH4++H2O��Al3++3NH3��H2O =Al(OH)3��+3NH4+���ʴ�Ϊ��H++NH3H2O=NH4++H2O��Al3++3NH3��H2O =Al(OH)3��+3NH4+��
(4)���������Ҫ�ɷ���Al2O3��������Fe2O3��SiO2�����ʣ�����������������������������Ʒ�Ӧ����ƫ�����ƺ����ƣ���Ӧ�ķ���ʽΪ��Al2O3+2NaOH�T2NaAlO2+H2O��SiO2+2NaOH�TNa2SiO3+H2O���ʴ�Ϊ��Al2O3+2NaOH�T2NaAlO2+H2O��SiO2+2NaOH�TNa2SiO3+H2O��
(5)��Һa�к���ƫ��������ӡ���������ӣ�������Һa��ͨ�������CO2���������������������������Ȼ��ֱ�ӽ��õ�������b���պ�õ��Ĺ����к����������Ͷ������裬��Al2O3�к���SiO2���ʣ��ʴ�Ϊ����������Al2O3�к���SiO2���ʣ�
(6)��״���£�2.24L���������ʵ���Ϊ![]() =0.1mol��������ڵ����������Ʊ�����������������������������������������ӦΪ2O2--4e-=O2����ת��0.4mol���ӣ���ת�Ƶ���0.4NA���ʴ�Ϊ��0.4NA��
=0.1mol��������ڵ����������Ʊ�����������������������������������������ӦΪ2O2--4e-=O2����ת��0.4mol���ӣ���ת�Ƶ���0.4NA���ʴ�Ϊ��0.4NA��

 ������ѧ���̲���ȫ���ϵ�д�
������ѧ���̲���ȫ���ϵ�д� ������ʱ����ҵ����ϵ�д�
������ʱ����ҵ����ϵ�д�