��Ŀ����
ij��Ӧ�з�Ӧ�����������У�FeCl2��FeCl3��CuCl2��Cu��
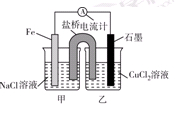
��1����������Ӧ��Ƴɵ�ԭ�����ͼ����ʾ����ش��������⣺

��ͼ��X��Һ��_____________________________��
��ʯī�缫�Ϸ����ĵ缫��ӦʽΪ__________________________________________��
��ԭ��ع���ʱ�������е�____________(�K������Cl����)���Ͻ���X��Һ�С�
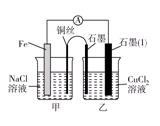
��2����������Ӧ��Ƴɵĵ�����ͼ����ʾ�����ձ��н��������ӵ����ʵ��������ת�Ƶ����ʵ����ı仯��ϵ��ͼ������ش��������⣺
��M��__________����
��ͼ���еĢ�����______________�ı仯��
�۵�����ת��Ϊ2 molʱ�������ձ��м���________ L 5 mol��L��1 NaOH��Һ����ʹ���еĽ��������ӳ�����ȫ��
��3��������Ҫ�������������(Na2FeO4)��һ����������ˮ�����������кܶ��ŵ㡣
�ٸ���������������֮һ�ǵ�ⷨ����ԭ��ΪFe��2NaOH��2H2O=Na2FeO4��3H2��������ʱ�����ĵ缫��Ӧʽ��__________________________________��
�ڸ���������������֮������ǿ���Խ�������NaClO����Fe(OH)3���ɸ������ơ��Ȼ��ƺ�ˮ���÷�Ӧ�����ӷ���ʽΪ_______________________________________��
��1�� ��FeCl3��FeCl2 ��Fe3++e-=Fe2+��K�� ��1�֣�
��2���ٸ���1�֣� ��Fe2������2.8
��3��
���������������1���ٲ��շ�Ӧ���Է��ԣ�Cuֻ�ܺ�FeCl3��Ӧ����Ϊ������Fe3++e-=Fe2+���������������ƶ�����2������ͼ��������ӵĹ�ϵ�����Է��֢�ʱͭ�ı仯��������Cu����������ʯī�������������� ���ӹ�ϵ��1:1�������Ƴ���Fe2+��������Ϊ2molʱ����������Cu2+ 1mol,Fe3+ 2mol Fe2+ 3mol.����ҪOH- 14mol,��Һ���Ϊ2.8L����3����Fe��8OH����6e��=FeO42-��4H2O��2Fe(OH)3��3ClO����4OH��=2=FeO42-��3Cl����5H2O
���㣺�绯ѧ��ػ���֪ʶ�����õ��ӣ����ԣ�ԭ���غ����֪ʶ��д�绯ѧ��Ӧ����ʽ��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
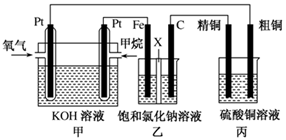
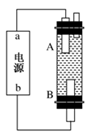
��˼ά������ҵϵ�д��縡ѡ���۷�����������ˮ�Ĺ���ԭ����ͼ��ʾ������˵������ȷ����
A�����缫�ĵ缫��ӦʽΪ�� |
B��ͨ�˿�����ʯī�缫�ĵ缫��ӦʽΪ |
| C�������ʯī�缫����44. 8L����״�������壬����������0. 5mol |
| D��Ϊ��ǿ��ˮ�ĵ���������������ˮ�м���������ҵ��ʳ�� |
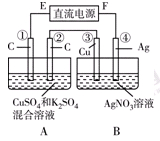
ij��ѧ��ȤС������ͼװ�õ��CuSO4��Һ���ⶨͭ����Է���������
��1����ʵ���вⶨ�ڱ�״���·ų������������VL��A����ֱ����Դ��__________ (�����������������
��2����ʼһ��ʱ�����U�ι��пɹ۲쵽��������____________________________��
�������ӷ���ʽΪ ��
��3��ʵ���л���ⶨ��������_______________����д��ţ���
��A������������m g ��B������������m g
��4������ʵ������б�Ҫ����____________������ĸ����
| A���������ǰ�缫������ |
| B�����缫�ں�ɳ���ǰ������������ˮ��ϴ |
| C�����µ������е缫��������ͭ������ϴ������ |
| D���缫�ں�ɳ��صIJ����б��밴����ɡ�����һ�ٺ��һ�ٳ��ء����� |
��5��ͭ�����ԭ������Ϊ��_______________________���ú���m��V�ļ���ʽ��ʾ����
��6������ü��ԣ�KOHΪ����ʣ��״�ȼ�ϵ����Ϊ��Դ����ʵ�飬�ŵ�ʱ�����ĵ缫��ӦʽΪ ��
��ҵ����﮻�ʯ��Li2O��A12O3��4SiO2��������Ca��MgԪ�أ�Ϊԭ������̼��ﮡ��䲿�ֹ����������£�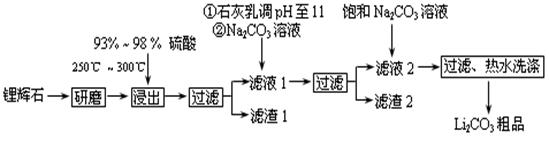
��֪����Li2O��Al2O3��4SiO2 + H2SO4(Ũ) Li2SO4 + Al2O3��4SiO2��H2O��
Li2SO4 + Al2O3��4SiO2��H2O��
| T/�� | 20 | 40 | 60 | 80 |
| S(Li2CO3)/g | 1.33 | 1.17 | 1.01 | 0.85 |
| S(Li2SO4)/g | 34.2 | 32.8 | 31.9 | 30.7 |

��2����֪����2����Ҫ�ɷ���Mg(OH)2��CaCO3��д����������2��Ӧ�����ӷ���ʽ�� ��
��3������Һ2�м��뱥��Na2CO3��Һ�����˺��á���ˮϴ�ӡ���ԭ���� ��
��4����ҵ�ϣ���Li2CO3��Ʒ�Ʊ��ɸߴ�Li2CO3�IJ��ֹ������¡�
�ٽ��ֲ�ƷLi2CO3�������������۵�����Һ��LiOH��Һ������Һ������������ѡ���Ĥ�������ö��Ե缫��⡣�����ĵ缫��Ӧʽ�� ��
�ڵ������ƷLiOH��Һ�м������NH4HCO3��Һ����Li2CO3��Ӧ�Ļ�ѧ����ʽ�� ��