��Ŀ����
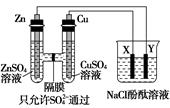
ij��ѧ��ȤС������ͼװ�õ��CuSO4��Һ���ⶨͭ����Է���������
��1����ʵ���вⶨ�ڱ�״���·ų������������VL��A����ֱ����Դ��__________ (�����������������
��2����ʼһ��ʱ�����U�ι��пɹ۲쵽��������____________________________��
�������ӷ���ʽΪ ��
��3��ʵ���л���ⶨ��������_______________����д��ţ���
��A������������m g ��B������������m g
��4������ʵ������б�Ҫ����____________������ĸ����
| A���������ǰ�缫������ |
| B�����缫�ں�ɳ���ǰ������������ˮ��ϴ |
| C�����µ������е缫��������ͭ������ϴ������ |
| D���缫�ں�ɳ��صIJ����б��밴����ɡ�����һ�ٺ��һ�ٳ��ء����� |
��5��ͭ�����ԭ������Ϊ��_______________________���ú���m��V�ļ���ʽ��ʾ����
��6������ü��ԣ�KOHΪ����ʣ��״�ȼ�ϵ����Ϊ��Դ����ʵ�飬�ŵ�ʱ�����ĵ缫��ӦʽΪ ��
��1���� ��2��A����֣�B�������ݲ�������Һ��ɫ��dz2Cu2����2H2O 2Cu��O2����4H��
2Cu��O2����4H��
��3���� ��4��ABDE ��5��11.2m/V ��6��CH3OH��8OH����6e��=CO32����6H2O
���������������1�����O2�����ΪVL��A��Ϊ������
��2������ΪA����֣�B�������ݲ�������Һ��ɫ��dz���缫��ӦʽΪ2Cu2����2H2O 2Cu��O2����4H����4���缫��������Cu����������Ҫ���£�ֻ��Ҫ�Ƴ�A�����������������ʲ���ҪC��IJ�����
2Cu��O2����4H����4���缫��������Cu����������Ҫ���£�ֻ��Ҫ�Ƴ�A�����������������ʲ���ҪC��IJ�����
��5�� ��ͭ��Ħ������ΪMmol/g 2Cu��O2
2M 1
m V/22.4
���M=11.2m/V
���㣺������ԭ������������֪ʶ��

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д����հ�ɽ��������������ɽ���ڣ�������½���ɽһ�������������׳ơ������͡�������������Ҫ�Ŀ���֮һ�������б���Ϊ����ʯ�����������ߣ�����ұ���̸ֵ���Ҫԭ�ϡ�����ʯ��Ҫ�ɷ��д�����Fe3O4��������FeCO3���̿�MnO2��MnCO3��ʯ��Mg3Si3O7(OH)4�ȡ���ҵ�Ͻ�����ʯ����������������Ĥ��ⷨ���¼�����ȡ�����̲��Ƶ���ɫ��Чˮ��������K2FeO4������ҵ�������£�
��1����ҵ��Ϊ���ϡ�����ȡЧ��һ���ȡ�Ĵ�ʩ�ǣ�����д���ַ�����
�� ��
��2��ʯ��ѧʽΪMg3Si3O7(OH)4Ҳ���Ա�ʾ����������ʽ�����������ʽΪ ��
��3����֪��ͬ����������������������������pH���±���
| ���� | Fe3+ | Al3+ | Fe2+ | Mn2+ | Mg2+ |
| ��ʼ������pH | 2.7 | 3.7 | 7.0 | 7.8 | 9.3 |
| ��ȫ������pH | 3.7 | 4.7 | 9.6 | 9.8 | 10.8 |
���̢��мӰ�ˮ������Һ��pH����6��������B�ijɷ� ��
��4������Һ����Mn2+��ʽ���ڣ�������A����MnO2ԭ�� ��
��5�����װ���м�ͷ��ʾ��Һ���������ƶ��ķ�����A�缫��ֱ����Դ�� ����ʵ�������У�������ϡ����Ϊ���Һ�������ĵ缫��ӦʽΪ ��

��6����������Ӧ��������ɫ��Чˮ�����������ӷ���ʽ ��
K2FeO4����Ϊ��ɫ��Чˮ��������ԭ���� ��




 ��
��


