��Ŀ����
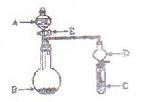
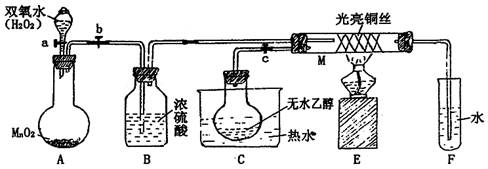
��14�֣���ͼΪʵ������ȡ�����������Cl2�������м���Cl2����ʵ���װ�á�����Eƿ�з��и����ɫ������F��Ϊͭ����F�Ҷ˳����ܿڸ���������֬�ޡ�

�Իش�
��1��A�������Լ�Ϊ ��B�������Լ�Ϊ �����߷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϊ�õ����﴿����������һ����Cƿ�м��� �Լ���Dƿ�м��� �Լ���
��3��Eƿ������Ϊ �� F�з�Ӧ�Ļ�ѧ����ʽΪ ��
��4��H��Ӧ������Լ�Ϊ ���������� ��
�仯ѧ����ʽΪ ��

�Իش�
��1��A�������Լ�Ϊ ��B�������Լ�Ϊ �����߷�Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϊ�õ����﴿����������һ����Cƿ�м��� �Լ���Dƿ�м��� �Լ���
��3��Eƿ������Ϊ �� F�з�Ӧ�Ļ�ѧ����ʽΪ ��
��4��H��Ӧ������Լ�Ϊ ���������� ��
�仯ѧ����ʽΪ ��
��14�֣�
��1�� Ũ���� (1��) MnO2 (1��) MnO2+4HCl(Ũ)="===" MnCl2+Cl2+2H2O (2��)
��2�� ����NaCl��Һ(1��) Ũ���� (1��)
��3�� ��ɫ��ȥ (1��) Cu+Cl2 ==== CuCl2 (2��)
��4�� NaOH��Һ(1��) ���ն�����������ֹ��Ⱦ���� (2��)
Cl2+2NaOH ==== NaCl+NaClO+H2O (2��)
��1�� Ũ���� (1��) MnO2 (1��) MnO2+4HCl(Ũ)="===" MnCl2+Cl2+2H2O (2��)
��2�� ����NaCl��Һ(1��) Ũ���� (1��)
��3�� ��ɫ��ȥ (1��) Cu+Cl2 ==== CuCl2 (2��)
��4�� NaOH��Һ(1��) ���ն�����������ֹ��Ⱦ���� (2��)
Cl2+2NaOH ==== NaCl+NaClO+H2O (2��)
��1����ʵ��װ��Ϊ������������A�������Լ�ΪŨ���ᣬB�������Լ�ΪMnO2���䷴Ӧ�Ļ�ѧ����ʽΪ��MnO2+4HCl(Ũ)="===" MnCl2+Cl2+2H2O��
��2��Ϊ�õ����﴿����������һ����Cƿ�м��뱥��NaCl��Һ��Dƿ�м���Ũ���
��3��Eƿ������Ϊ��ɫ��ȥ��F�з�Ӧ�Ļ�ѧ����ʽΪCu+Cl2 ==== CuCl2��
��4��H��Ӧ������Լ�ΪNaOH��Һ�������������ն�����������ֹ��Ⱦ��������Ӧ�ķ���ʽΪ��Cl2+2NaOH ==== NaCl+NaClO+H2O
��2��Ϊ�õ����﴿����������һ����Cƿ�м��뱥��NaCl��Һ��Dƿ�м���Ũ���
��3��Eƿ������Ϊ��ɫ��ȥ��F�з�Ӧ�Ļ�ѧ����ʽΪCu+Cl2 ==== CuCl2��
��4��H��Ӧ������Լ�ΪNaOH��Һ�������������ն�����������ֹ��Ⱦ��������Ӧ�ķ���ʽΪ��Cl2+2NaOH ==== NaCl+NaClO+H2O

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
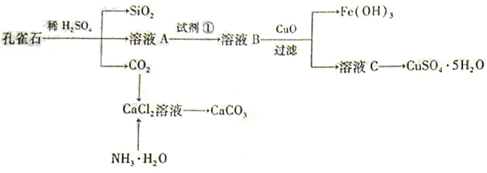
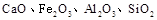 ��ɣ�����ʯ��ʯ��ȡ�Ȼ��ƺ���������ʵ�鲽�����£�
��ɣ�����ʯ��ʯ��ȡ�Ȼ��ƺ���������ʵ�鲽�����£�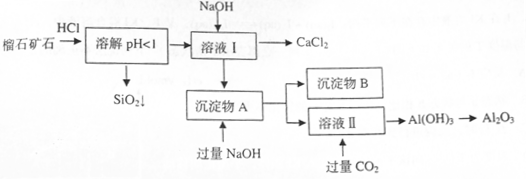
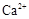 �⣬�����еĽ��������� ��
�⣬�����еĽ��������� ��
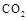 ���岢ͨ����ҺII�У����û�г������������ܵ�ԭ�� ��Ϊ���ܲ���������ͬѧ��ͼIװ�ý����˸Ľ����Ľ��ķ���Ϊ ��
���岢ͨ����ҺII�У����û�г������������ܵ�ԭ�� ��Ϊ���ܲ���������ͬѧ��ͼIװ�ý����˸Ľ����Ľ��ķ���Ϊ ��
 2H++ Fe��OH��2��25��ʱ��ƽ�ⳣ��
2H++ Fe��OH��2��25��ʱ��ƽ�ⳣ�� 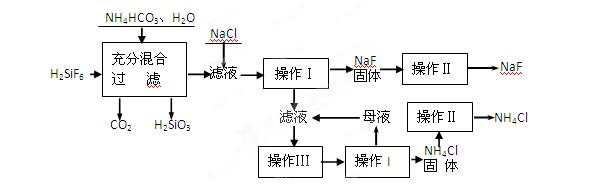
 .3
.3
 ����������ҺA��Fe3+������Լ�Ϊ��������������ţ���
����������ҺA��Fe3+������Լ�Ϊ��������������ţ���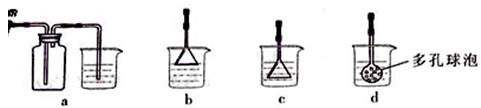
 �����ⶨ��ҺA��Fe2+��Ũ�ȣ���Ҫ������ƿ����ij����Һ������ʱ��������ƿ�Ŀ̶��ߣ���ʹ���Ƶ�Ũ�� �����ƫ�ߡ�����ƫ�͡�������Ӱ�족����
�����ⶨ��ҺA��Fe2+��Ũ�ȣ���Ҫ������ƿ����ij����Һ������ʱ��������ƿ�Ŀ̶��ߣ���ʹ���Ƶ�Ũ�� �����ƫ�ߡ�����ƫ�͡�������Ӱ�족����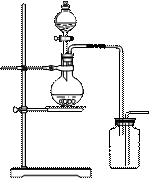
 ���������ӡ�
���������ӡ�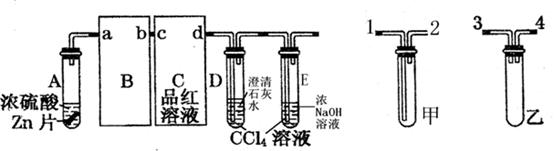
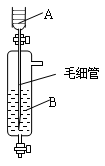
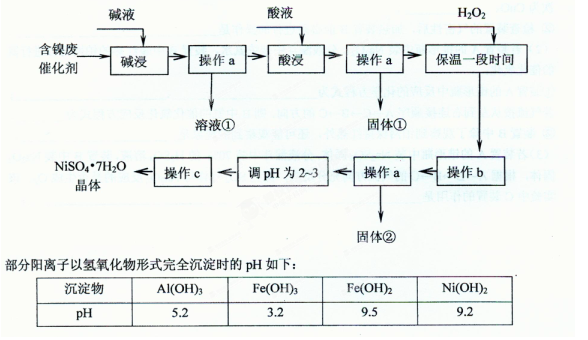
 ���������ʵ�顣��ʵ��װ������ͼ��ʾ��
���������ʵ�顣��ʵ��װ������ͼ��ʾ��