��Ŀ����
����Ŀ��
(1)�ڱ�״���£������ʢ�44.8LH2����24gCH4����1molH2O����3.01��1023��N2����������������______������ţ���ͬ������������������________������������______�������С����______���ܶ���С�����˳��Ϊ________________��
(2)0.5molij����A��������40g��A��Ħ������Ϊ_________��
(3)����֮��Ϊ8��7����������O2��CO���������֮��Ϊ_______________����ԭ����֮��Ϊ____________����ͬ�����µ����֮��Ϊ________��
(4) 4.8g̼��һ������������ȼ�գ���Ӧ������CO��CO2��������Ϊ12.8g������ͬ��ͬѹ�£����ɵ�CO��CO2�������Ϊ__________��
(5)��NaHS��MgSO4��NaHSO3��ɵĻ�����У���Ԫ�ص���������Ϊa��������������Ԫ�ص���������Ϊ_______________��
���𰸡��� �� �� �� �٢ڢܢ� ���� ��<��<��<�ۣ� 80 g��mol��1 1��1 2��1 1��1 3��1 1-1.75a��
��������
��1���������״���£�![]() ��
��![]() ��
��![]() ��
��![]() �����ʵ������ٸ���
�����ʵ������ٸ���![]() ��
��![]() ��
��![]() �������ʽ������ʽ��������������ܶȡ�
�������ʽ������ʽ��������������ܶȡ�
��2������![]() ����Ħ��������
����Ħ��������
��3�������![]() ��
��![]() �����ʵ���֮�ȣ����������֮�ȡ���ԭ����֮�ȣ�ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
�����ʵ���֮�ȣ����������֮�ȡ���ԭ����֮�ȣ�ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
��4������̼�����ʵ������������ɵ�![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ��
��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ���з��������
����������![]() ��
��![]() ��ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
��ͬ��ͬѹ�����֮�ȵ������ʵ���֮�ȡ�
��5��������������ɣ���֪![]() ��
��![]() �����ʽ������24����������������
�����ʽ������24����������������![]() ����
����![]() �Ĺ�ϵ�����
�Ĺ�ϵ�����![]() ��
��![]() ��
��![]() ��
��![]() Ԫ�ص���������������
Ԫ�ص���������������![]() Ԫ�ص�����������
Ԫ�ص�����������
��1��
��״̬�� | ���ʵ��� | ������ | ������ | ���� | ��� | �ܶ� |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
�����������Ǣ٣��������������������������Ǣڣ������С�������������ܶ���С����� < �� < �� < ��
��2��![]()
��3��![]() ��
��![]() �����ʵ���֮��Ϊ��
�����ʵ���֮��Ϊ��![]() ���������֮��Ϊ��
���������֮��Ϊ��![]() ����ԭ����֮��Ϊ��
����ԭ����֮��Ϊ��![]() ��ͬ�����µ����֮�ȵ������ʵ���֮�ȣ�Ϊ
��ͬ�����µ����֮�ȵ������ʵ���֮�ȣ�Ϊ![]()
��4��![]() ̼�����ʵ���Ϊ
̼�����ʵ���Ϊ![]() �������ɵ�
�������ɵ�![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ��
��![]() �����ʵ���Ϊ
�����ʵ���Ϊ![]() ����
����![]() �����
�����![]() ��
��![]() ����ͬ��ͬѹ�£�CO��CO2�����֮�ȵ������ʵ���֮�ȣ���
����ͬ��ͬѹ�£�CO��CO2�����֮�ȵ������ʵ���֮�ȣ���![]() ��
��
��5����![]() ��
��![]() ����һ�����壬���ǵ����ʽ������24����������������S����
����һ�����壬���ǵ����ʽ������24����������������S����![]() �Ĺ�ϵ����Ԫ�ص���������Ϊa�����������Ǿ���
�Ĺ�ϵ����Ԫ�ص���������Ϊa�����������Ǿ���![]() ������
������
![]()

����Ŀ���ҹ�����ר�Һ�°�ġ������Ƽ����Ϊ�����Ƽҵ������ͻ�����ף����Ա���ʳ��ˮ��NH3��CO2Ϊԭ�����Ƶ�NaHCO3����������������ش��������⣺
ij̽���С����������Ƽ�ԭ��������̼�����Ƶ��Ʊ�ʵ�飬ͬѧ�ǰ�������Ʒ���ʵ�顣һλͬѧ��������̼����ͨ�뺬���ı���ʳ��ˮ���Ʊ�̼�����ƣ�ʵ��װ����ͼ��ʾ(ͼ�мг֡��̶��õ�����δ����)
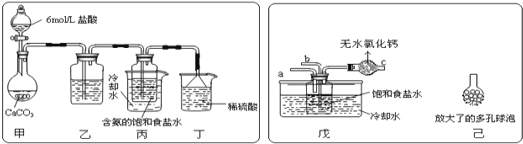
��1����װ����ϡ�����������____��
��2����һλͬѧ��ͼ����װ�ã�����װ��δ����������ʵ�顣ʵ��ʱ�����ȴ�___��ͨ��NH3���塣
��3����ͬѧ��������װ�õ�b���¶����Ӽ�װ�ã�������_____��
��4����������г�������������ڲ�ͬ�¶��µ��ܽ�����ݣ�g/100gˮ����
0�� | 10�� | 20�� | 30�� | 40�� | 50�� | |
NaCl | 35.7 | 35.8 | 36.0 | 36.3 | 36.6 | 37.0 |
NaHCO3 | 6.9 | 8.1 | 9.6 | 11.1 | 12.7 | 14.5 |
NH4Cl | 29.4 | 33.3 | 37.2 | 41.4 | 45.8 | 50.4 |
���ձ������ݣ������������װ����ʹ����ȴˮ���߱�ˮ��ԭ��____��
��5����С��ͬѧΪ�˲ⶨ�������þ����̼�����ƵĴ��ȣ����辧���в���̼�������ʣ����������ָ����������Ϊag���ٽ�������ȵ��������ٱ仯ʱ���������÷�ĩ����Ϊmg��Ȼ�������ͼ��ʾʵ�飺
![]()
���ڲ������У�Ϊ���жϼ����Ȼ�����Һ�Ƿ������������ȷ����___������ĸ����
a �ڼ����Ȼ�����Һ�������ã�����Һ�м������������Ȼ�����Һ
b �ڼ����Ȼ�����Һ�������ã�����Һ���ټ�������̼������Һ
c �ڼ����Ȼ�����Һ�������ã�ȡ�ϲ���Һ�ټ�������̼������Һ
�����þ�����̼�����ƵĴ���Ϊ___��
����Ŀ��Ϊ��ǿ������ʴ�ԣ�����Ǧ����Ϊ���Դ����Al��������Pb�����������ϡ���ᣬʹ�����������Ĥ�����䷴Ӧԭ�����£� ��أ� Pb(s) + PbO2(s) + 2H2SO4(aq) =2PbSO4(s) + 2H2O(l)��
���أ�2Al+3O2![]() Al2O3+3H2���������У������ж���ȷ���ǣ� ��
Al2O3+3H2���������У������ж���ȷ���ǣ� ��
��� | ���� | |
A | H+����Pb�缫 | H+����Pb�缫 |
B | ÿ����3molPb | ����2molAl2O3 |
C | ������PbO2+4H++2e��=Pb2++2H2O | ������2Al+3H2O-6e��=Al2O3+6H+ |
D |
|
|