��Ŀ����
����Ŀ������̼��ˮ�����Ʊ�ˮú���ĺ��ķ�ӦΪ��C(s)��H2O(g)H2(g)��CO(g)
(1)��֪̼(ʯī)��H2��CO��ȼ���ȷֱ�Ϊ393.5kJ��mol��1��285.8kJ��mol��1��283kJ��mol��1����֪H2O(l)=H2O(g)����H����44kJ��mol��1����C(s)��H2O(g)CO(g)��H2(g)����H��___��
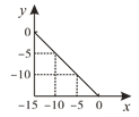
(2)��ij�¶��£������Ϊ1L�ĺ����ܱո��������м�����������̿��������1mol H2O(g)����������Ӧ����Ӧʱ����������������ѹǿ�����������
ʱ��/min | 0 | 10 | 20 | 30 | 40 |
��ѹǿ/100kPa | 1.0 | 1.2 | 1.3 | 1.4 | 1.4 |
��ƽ��ʱ�����������������ʵ���Ϊ________mol��H2O��ת����Ϊ________��
�����¶��·�Ӧ��ƽ���ѹ����Kp��________kPa(�������2λ��Ч����)��
(3)����25��������㶨��1L������Ͷ����������̿��������壬�������淴ӦC��H2O(g)CO��H2���ѽ���ƽ�⣬��40 minʱ�ٳ���һ����H2��50minʱ�ٴδﵽƽ�⣬��Ӧ�����и����ʵ�Ũ����ʱ��仯��ͼ��ʾ��
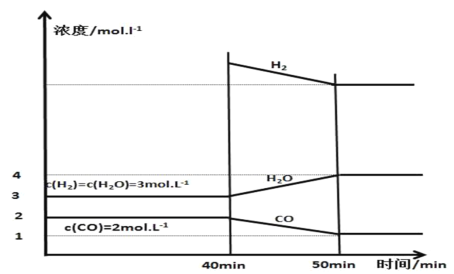
��40minʱ���ٳ����H2�����ʵ���Ϊ________mol��
��40��50 min��H2��ƽ����Ӧ����Ϊ________mol��L��1��min��1��
(4)���͵������������ڽ����ơ�������Ͷ�����(Na2Sx)�ֱ���Ϊ�����缫�ķ�Ӧ�����Al2O3�մ�(�ɴ���Na��)Ϊ����ʣ���ԭ����ͼ��ʾ��
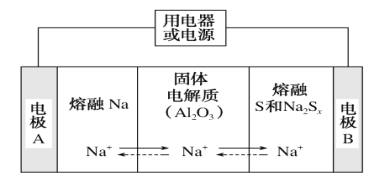
���ŵ�ʱ���缫AΪ________����S����________��Ӧ�����������ԭ������
�����ʱ���ܷ�ӦΪNa2Sx=2Na��Sx(3<x<5)��Na���ڵ缫��ֱ����Դ________�������������ĵ缫��ӦʽΪ_________��
���𰸡���131.3 kJ��mol��1 1.4 40% 27 6 0.1 �� ��ԭ �� Sx2--2e-=Sx
��������
(1)��֪̼(ʯī)��H2��CO��ȼ���ȷֱ�Ϊ393.5kJ��mol��1��285.8kJ��mol��1��283kJ��mol��1����
��C(s)��O2(g)��CO2(g)����H����393.5kJ��mol��1
��![]() O2(g)��H2(g)��H2O(l)����H����285.8kJ��mol��1
O2(g)��H2(g)��H2O(l)����H����285.8kJ��mol��1
��CO(g)��![]() O2(g)��CO2(g)����H����283kJ��mol��1
O2(g)��CO2(g)����H����283kJ��mol��1
��H2O(l)=H2O(g)����H����44kJ��mol��1
���ݸ�˹���ɿ�֪�����������������õ�C(s)��H2O(g)![]() CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��
(2)���ݷ���ʽ��֪
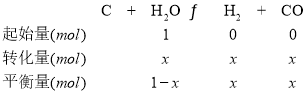
ѹǿ֮�������ʵ���֮�ȣ���(1��x+x+x):1��1.4:1�����x��0.4��
���������Ϸ�����֪ƽ��ʱ�����������������ʵ���Ϊ1.4mol��H2O��ת����Ϊ40%��
�����¶��·�Ӧ��ƽ���ѹ����Kp��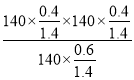 ��27kPa��
��27kPa��
(3)����50minʱH2�����ʵ���Ϊx mol���¶Ȳ���ƽ�ⳣ�����䣬�����ݻ���1L�������ͼ���֪ƽ�ⳣ��K=![]() �����x��8������CO�ı仯����1mol������40minʱ�ٳ����H2�����ʵ���Ϊ8mol+1mol��3mol��6mol��
�����x��8������CO�ı仯����1mol������40minʱ�ٳ����H2�����ʵ���Ϊ8mol+1mol��3mol��6mol��
�����������ı仯����1mol������40��50 min��H2��ƽ����Ӧ����Ϊ![]() ��0.1mol��L��1��min��1��
��0.1mol��L��1��min��1��
(4)���ŵ�ʱ��ʧȥ���ӣ���缫AΪ�������缫B����������S������ԭ��Ӧ��
���ŵ�ʱ��ʧȥ���ӣ���缫AΪ���������ʱ��Na���ڵ缫����������ֱ����Դ������������������ʧȥ���ӵ�������Ӧ��������ܷ�ӦΪNa2Sx=2Na��Sx(3��x��5)��֪�������缫��ӦʽΪSx2--2e-=Sx��