��Ŀ����
1��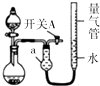 ijͬѧ���ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ������ش��������⣺
ijͬѧ���ͼ��ʾװ�÷ֱ����̽��ʵ�飨�г�װ������ȥ������ش��������⣺| ʵ�� | ҩƷ | ��ȡ���� | �������е�Һ�� |
| �� | Cu��ŨHNO3 | H2O | |
| �� | CaO���塢Ũ��ˮ | NH3 | |
| �� | ��þ��ϡH2SO4 ��������[���ʲ������ᷴӦ] | H2 | H2O |
��2������װ�õ������Եķ����ǣ��رտ���A�ͷ�Һ©������������ƿ���������Ҷ�Һ�����ߣ�˵�����������ã�
��3����ͬѧ��Ϊʵ��ٿ�ͨ���ռ�����NO2������������̽��Cu��Ʒ�Ĵ��ȣ������Ƿ���з���ǡ�����ʵ�������ƿ�ڷ�Ӧ�����ӷ���ʽΪ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��4��ʵ����У��������е�Һ�������D��
A��H2O B������NaHCO3��ҺC������Na2CO3��Һ D��CCl4
��5����ʵ��Ӧ�������ܶ�ζ���������ʱӦע�⣺
�ٻָ������£��ڱ�����������Һ����������Һ��Һ����ƽ���������밼Һ����ʹ���ƽ��
��6��ʵ��ۻ���������ݣ���������������ѻ���ɱ�״������
| ��� | þ�������ʣ����� | �����ܵ�һ�ζ��� | �����ܵڶ��ζ��� |
| 1 | 0.5g | 10.0mL | 346.5mL |
| 2 | 0.5g | 10.0mL | 335.0mL |
| 3 | 0.5g | 10.0mL | 345.5mL |
���� ��1������aΪ����ܣ�
��2����������������������رտ���A�ͷ�Һ©������������ƿ��������Һ�����ߣ�˵�����������ã�
��3������������ˮ��Ӧ�����²ⶨ�����������������Cu��Ũ���ᷴӦ��������ͭ������������ˮ��
��4����������Һ���ѡ����ǣ������岻��Ӧ�����ܽ�����壻
��5���������ܶ���ʱ�����ȵ�ʵ��װ�ûָ��������ٽ�����һ��������Ȼ�����������ʹ����Һ����ƽ��������ʱ�����밼Һ����ʹ���ƽ��
��6��ʵ����У���2�����1��3��ʵ����������������ϵͣ�Ӧ��ȥ��2�����ݣ����������ܵĵڶ��ζ���-��һ�ζ���=������������������õ�1��3�������������������ƽ��ֵ���ٸ��ݷ���ʽ�����þ��Mg�����������������Mg������������
��� �⣺��1������aΪ����ܣ��ʴ�Ϊ������ܣ�
��2���رտ���A�ͷ�Һ©������������ƿ���������Ҷ�Һ�����ߣ�˵�����������ã�
�ʴ�Ϊ������ƿ���������Ҷ�Һ�����ߣ�
��3������������ˮ��Ӧ�����²ⶨ������������������ʷ��������У�Cu��Ũ���ᷴӦ��������ͭ������������ˮ����Ӧ���ӷ���ʽΪ��Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
�ʴ�Ϊ����Cu+4H++2NO3-=Cu2++2NO2��+2H2O��
��4��ʵ����а�����������ˮ��������������Һ�岻��ѡ��ˮ��ˮ��Һ��ֻ��ѡ�Ͱ�������Ӧ�����Ȼ�̼���ʴ�Ϊ��D��
��5������PV=nRT��Ϊ��֤���������������ǵ�ʱ����ѹ�µ�������ڶ���ʱӦע�⣺�ٽ�ʵ��װ�ûָ������£���ʹ����������Һ����ƽ����ƽ˵������Һ���ϵ�ѹǿһ�������������������������ǵ�ʱ����ѹ�µ�������������밼Һ����ʹ���ƽ�����ӻ����ӻ������
�ʴ�Ϊ��������������Һ����������Һ��Һ����ƽ��
��6��ʵ����У���2�����1��3��ʵ����������������ϵͣ�Ӧ��ȥ��2�����ݣ������������Ϊ$\frac{��346.5-10��mL+��345.5-10��mL}{2}$=336mL=0.336L��
������0.346L��������Mg������Ϊx����
Mg+H2SO4�TMgSO4+H2��
24g 22.4L
x 0.336L
x=$\frac{24g��0.336L}{22.4L}$=0.36g
����þ����������Ϊ $\frac{0.36g}{0.5g}$��100%=72%
�ʴ�Ϊ��72%��
���� ���⿼�����ʺ����IJⶨ������ԭ���ǽ���ؼ����Ƕ�ѧ���ۺ������Ŀ��飬��Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�

| A�� | W��Y��Z����Ԫ�ص�����������ˮ���������������ǿ�һ����ǿ�� | |
| B�� | ZԪ���ڵ�3���ڵڢ�A�� | |
| C�� | W��Y��Z����Ԫ���������ǽ�����һ���Ƿǽ��� | |
| D�� | W��X��Y����Ԫ������������Ӧˮ����ļ���������ǿ |
| A�� | Si��B | B�� | N��Be | C�� | S��Mg | D�� | C��Al |
| A�� | ��AlCl3��Һ�м��������ˮ��Al3++3 NH3•H2O�TAl��OH��3��+3NH4+ | |
| B�� | ������������Һ�м���ϡ���3Fe2++NO3-+4H+�T3Fe3++2H2O+NO�� | |
| C�� | ̼�������Һ��������NaOH��Һ��Ϻ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | |
| D�� | AlCl3��Һ�еμ�������ˮ��Al3++4NH3•H2O�TAlO2-+4NH4++2H2O |
| A�� | ����β���з����Ĵ�ת����Ӧ��2NO+2CO$\frac{\underline{\;����\;}}{\;}$N2+2CO2 | |
| B�� | ��ҵ��������MnO2+4HCl��Ũ�� $\frac{\underline{\;\;��\;\;}}{\;}$MnCl2+Cl2��+2H2O | |
| C�� | ȼúʱ����ʯ��ʯ����SO2�ŷţ�2CaCO3+2SO2+O2$\frac{\underline{\;����\;}}{\;}$2CaSO4+2CO2 | |
| D�� | ��Na2CO3��Һ����ˮ���еIJ�����CaSO4��CaSO4+CO32-�TCaCO3+SO42- |
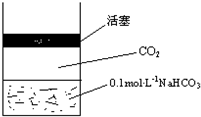 �ڳ����£�̼��ĵ��볣��Ka1=4.3��10-7��Ka2=5.6��10-11����ͼ��ʾ��װ���У����������ƣ�����˵������ȷ���ǣ�������
�ڳ����£�̼��ĵ��볣��Ka1=4.3��10-7��Ka2=5.6��10-11����ͼ��ʾ��װ���У����������ƣ�����˵������ȷ���ǣ�������| A�� | 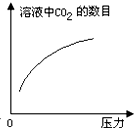 | B�� | 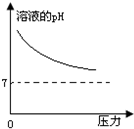 | C�� |  | D�� | 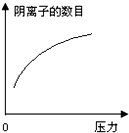 |
| A�� | �������백ˮ��Ӧ��Al3++3 OH-�TAl��OH��3�� | |
| B�� | ��������Һ��ͨ��CO2���壺SiO32-+H2O+CO2�TH2SiO3��+CO32- | |
| C�� | ����NH4Cl��Һ�м���NaOHŨ��Һ��NH4++OH-�TNH3��+H2O | |
| D�� | ����ͨ��FeCl2��Һ�У�2Fe2++Cl2�T2 Fe3++2Cl- |
��3000��Si��s��+3HCl��g��=SiHCl3��g��+H2��g�� �����ȷ�Ӧ��
��950��SiHCl3��g��+H2 ��g��=Si��s��+3HCl��g��
������������Ӧ�����������У�������ǣ�������
| A�� | ������Ӧ����������ԭ��Ӧ | |
| B�� | ������Ӧ�����û���Ӧ | |
| C�� | ������Ӧ���л�ѧ��������֮���ת�� | |
| D�� | ��Ӧ��2���Ƿ��ȷ�Ӧ |
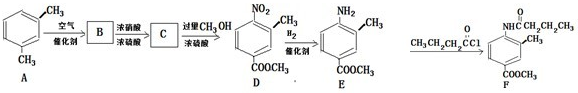
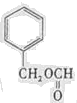
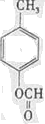
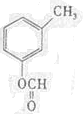
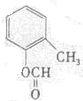 ��д�ṹ��ʽ����
��д�ṹ��ʽ����