��Ŀ����
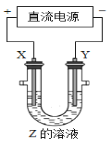
����Ŀ��ʵ���ҿ��������������Ʊ���ˮ���Ȼ�����SnCl4����SnCl4�ӷ���������ˮ�⣬Cl2��������SnCl4���Ʊ�ԭ����ʵ��װ��ͼ���£�
Sn(s)+2Cl2(g)=SnCl4(l) ��H = �C511kJmol��1

�����õ����й��������£�
���� | Sn | SnCl4 | CuCl2 |
�۵�/�� | 232 | -33 | 620 |
�е�/�� | 2260 | 114 | 993 |
�Ʊ������У����������ģ�������������ʱ��Ӧ���в�����������SnCl4Һ��������ڸ߶�ʱ��Һ̬���ᆳ����������ƿ���ش��������⣺
��1��a�ܵ�������________��
��2��A�з�Ӧ�����ӷ���ʽ��________��
��3��D��������________��
��4��E����ȴˮ��������________��
��5��β������ʱ����ѡ�õ�װ����________������ţ���

��6�������к�ͭ������E�в���CuCl2������Ӱ��F�в�Ʒ�Ĵ��ȣ�ԭ����________��
��7��SnCl4��Ʒ�к���Cl2������ʱ����������м������ɵô�����SnCl4����������в���Ҫ�õ���������________������ţ���
A��������ƿ B���¶ȼ� C�������� D�������� E������ƿ
���𰸡�ƽ��ѹǿ��ʹŨ�����ܹ�˳������ 2MnO4��+10Cl��+16H+=2Mn2++5Cl2��+8H2O ʹ������ָ��� �������Ȼ�����������ʧ�����²����½� �� CuCl2�۵�ϸߣ����������Ȼ���Һ����� E
��������
��1��a�ܽ���Һ©��Һ��������ͨ����ƽ��ѹǿ��ʹŨ�����ܹ�˳�����µ����ã�
��2��A�еķ�ӦΪ������غ�Ũ��������������Ӧ�����Ȼ��̡��Ȼ��ء�������ˮ�������غ��ϵ��ƽ��д�����ӷ���ʽ��
��3��DΪʢ��Ũ�����ϴ��ƿ������������������������Ϣ��֪��SnCl4������ˮ�⣬��D��������ʹ������ָ��
��4������Ϣ��֪��SnCl4�ӷ�����E����ȴˮ�������DZ������Ȼ�����������ʧ�����²����½���
��5��β������ʱ��ѡ�õ�װ���в���ʹ��ˮ��Һ����Ϊˮ��������E��ʹSnCl4����ˮ�⣻
��6���ɱ������ݿ�֪��CuCl2�۵�ϸߣ����������Ȼ���Һ����������������к�ͭ������E�в���CuCl2������Ӱ��F�в�Ʒ�Ĵ��ȣ�
��7�������������Ҫ�õ���������������ƿ���¶ȼơ������ܺͽ��������Դ˷�����
��1��a�ܽ���Һ©��Һ��������ͨ����ƽ��ѹǿ��ʹŨ�����ܹ�˳�����µ����ã�
�ʴ�Ϊ��ƽ��ѹǿ��ʹŨ�����ܹ�˳�����£�
��2��A�еķ�ӦΪ������غ�Ũ��������������Ӧ�����Ȼ��̡��Ȼ��ء�������ˮ�����ӷ���ʽΪ2MnO4��+10Cl��+16H+=2Mn2++5Cl2��+8H2O��
�ʴ�Ϊ��2MnO4��+10Cl��+16H+=2Mn2++5Cl2��+8H2O��
��3��DΪʢ��Ũ�����ϴ��ƿ������������������������Ϣ��֪��SnCl4������ˮ�⣬��D��������ʹ������ָ��
�ʴ�Ϊ��ʹ������ָ��
��4������Ϣ��֪��SnCl4�ӷ�����E����ȴˮ�������DZ������Ȼ�����������ʧ�����²����½���
�ʴ�Ϊ���������Ȼ�����������ʧ�����²����½���
��5��β������ʱ��ѡ�õ�װ���в���ʹ��ˮ��Һ����Ϊˮ��������E��ʹSnCl4����ˮ�⣬�ʲ�ѡ�ס�����Ӧѡ�ң�
�ʴ�Ϊ���ң�
��6���ɱ������ݿ�֪��CuCl2�۵�ϸߣ����������Ȼ���Һ����������������к�ͭ������E�в���CuCl2������Ӱ��F�в�Ʒ�Ĵ��ȣ�
�ʴ�Ϊ��CuCl2�۵�ϸߣ����������Ȼ���Һ�������
��7�������������Ҫ�õ���������������ƿ���¶ȼơ������ܺͽ���������������ƿ���ʲ�ѡE��
�ʴ�Ϊ��E��

 ���źþ���Ԫ����ĩ��ϵ�д�
���źþ���Ԫ����ĩ��ϵ�д� һ����ʦȨ����ҵ��ϵ�д�
һ����ʦȨ����ҵ��ϵ�д�����Ŀ�����к͵ζ����ⶨij�ռ�Ĵ��ȡ���2.5g������������(�������ᷴӦ)�Ĺ����ռ���Ʒ���Ƴ�250mL��Һ������ʵ��ش��������⣺
��1���ζ�
����____________����������ȡ20.00mL����Һ������ƿ�У��ٵμ�2�η�̪��Һ��
����____________��������ʢװ0.2000 mol��L��1�����Һ��ʢװǰ���____________����ֹ��ҺŨ�Ƚ��͡�
�۵ζ��������۾�Ӧע��_______________���ζ��յ������Ϊ��_______________________________________________________��
��2���й����ݼ�¼���£�
�ζ���� | ����Һ�����(mL) | �ζ�ǰ��mL�� | �ζ�����mL�� | �����������Һ��ƽ�����(mL) |
1 | 20.00 | 0.50 | 20.70 | V |
2 | 20.00 | 6.00 | 26.00 |
����V=________ml
��3�����ȼ��㣺NaOH��Һ��Ũ��Ϊ________ mol��L��1���ռ���Ʒ�Ĵ���Ϊ________��
��4�����ж����¼���������ռ�Ȳⶨ��Ӱ�죨�ƫ����ƫС������Ӱ�족����
����������ˮ��ϴ��ƿ�����ʹ�ⶨ���________��
�����ζ�ǰ�ζ��ܼ��������ݣ��ζ���������ʧ�����ʹ�ⶨ���________��
�����ռ���ָʾ���ֲ�����ɫ�б仯��ֹͣ�ζ������ʹ�ⶨ���________��
�ܶ���ʱ�����ζ�ǰ���ӣ��ζ����ӣ����ʹ�ⶨ���________��
����Ŀ����ʹ������к͵ζ����ⶨ���۰״�(��Ҫ�ɷ���CH3COOH)��������(g��100 mL��1)����֪CH3COOH + NaOH===CH3COONa + H2O �յ�ʱ������Һ�ʼ��ԡ�
��.ʵ�鲽�裺
(1)����Һ����ȡ10.00 mLʳ�ð״ף����ձ�����ˮϡ�ͺ�ת�Ƶ�100mL__________(����������)�ж��ݣ�ҡ�ȼ��ô���״���Һ��
(2)��_____ȡ����״���Һ20.00 mL����ƿ�С�
(3)�μ�2��_____________��ָʾ����
(4)��ȡʢװ0.100 0 mol��L��1NaOH ��Һ�ļ�ʽ�ζ��ܵij�ʼ������ ���Һ��λ����ͼ��ʾ�����ʱ�Ķ���Ϊ________mL��
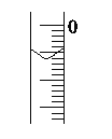
(5)�ζ�����__________________ʱ��ֹͣ�ζ�������¼NaOH��Һ���ն������ظ��ζ�3�Ρ�
��.ʵ���¼
ʵ�����ݣ�mL�� | 1 | 2 | 3 | 4 |
V(��Ʒ) | 20.00 | 20.00 | 20.00 | 20.00 |
V(NaOH)(����) | 15.95 | 15.00 | 15.05 | 14.95 |
��.���ݴ��������ۣ�
(1)�����������ݷ�����c(���۰״�)��________mol��L��1��
(2)�ڱ�ʵ��ĵζ������У����в�����ʹʵ����ƫС����_______(��д���)��
A����ʽ�ζ����ڵζ�ʱδ�ñ�NaOH��Һ��ϴ
B����ʽ�ζ��ܵļ����ڵζ�ǰ�����ݣ��ζ���������ʧ
C����ƿ�м������״���Һ���ټ�����ˮ
D����ƿ�ڵζ�ʱ����ҡ����������Һ�彦��
E. �ζ��յ����ʱ����