��Ŀ����
��֪W��X��Y��ZΪ������Ԫ�أ����ǵ�ԭ�Ӱ뾶��������W��Z��X��Y�ֱ�ͬ���壬Y��Zͬ���ڡ�X��Y���γ����ֳ������ۻ����Z����X�γ����ֳ��������ӻ�������������Ӹ����Ⱦ�Ϊ1��2������˵���������
| A��Y��Z��X��W��ԭ���������μ�С |
| B��X�ļ��⻯��ķе����Y�ļ��⻯��ķе� |
| C����W��X��Y��Z����Ԫ����ɵĻ����������ǿ���� |
| D��W��X�γɵĻ������W��Y�γɵĻ������и�Ԫ�������ȿ�����ͬ |
B
������������������֪�⼸��Ԫ�طֱ�Ϊ��W��H��X��O��Y��S��Z��Na��A��S��Na��O��H��ԭ���������μ�С����ȷ��B��X�ļ��⻯��H2O����֮����˴��ڷ��Ӽ�������������������H2S�ķ���֮��ֻ���ڷ��Ӽ�����������������DZȷ��Ӽ�������ǿ�Ķ�������������Կ˷������ĵ������ϸߣ���˷е�H2O����H2S������C����H��O��S��Na����Ԫ����ɵĻ�����NaHSO4��ǿ�����ʽ�ε������������H+��ʹ��Һ��ǿ���ԡ���ȷ��D��H��O�γɵĻ�����H2O2����H��S�γɵĻ�����H2S�и�Ԫ����������ͬ����ȷ��

��ϰ��ϵ�д�
�����Ŀ
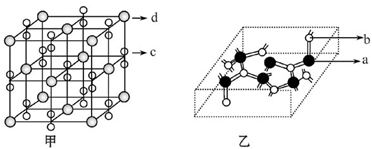

 g��cm��3�������ı߳�Ϊacm�����ӵ�����Ϊ__ mol-l���ú��ѡ�a��ʽ�ӱ�ʾ����
g��cm��3�������ı߳�Ϊacm�����ӵ�����Ϊ__ mol-l���ú��ѡ�a��ʽ�ӱ�ʾ����
