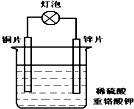
��Ŀ����
16�������������壨FeSO4•7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�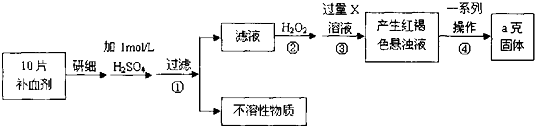
��ش��������⣺
��1��֤���������Һ�к���Fe2+�ķ������ȵμ�KSCN��Һ���ٵμ���ˮ���ù��̵�����Ϊ����Һ��dz��ɫ��ΪѪ��ɫ��
��2������ڼ������H2O2��Ŀ�ģ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ��������п�����ʣ�
��3��������з�Ӧ�����ӷ���ʽ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��
��4���������һϵ�д����IJ������裺���ˡ�ϴ�ӡ����ա���ȴ��������
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����0.07ag��
��6����С����Щͬѧ��Ϊ��KMnO4��Һ�ζ�Ҳ�ܽ�����Ԫ�غ����IJⶨ��
��5Fe2++MnO4-+8H+��5Fe3++Mn2++4H2O��
��ʵ��ǰ������Ҫ��ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ���������ձ�����ͷ�ι��⣬����250mL����ƿ��
������ʵ���е�KMnO4��Һ��Ҫ�ữ�������ữ������b��
a��ϡ���� b��ϡ���� c��ϡ���� d��Ũ����
�۵ζ����յ�ʱ����ɫΪ��ɫ��
��7��������ÿ��Ӧ����14mg���ҵ��������о���������ʳ����ȫ��ͨ�����ú�FeSO4•7H2O��Ƭ����������������������ÿ������ú�69.5mgFeSO4•7H2OΪƬ����
���� ������ͼ��֪����ʵ��ԭ��Ϊ����ҩƷ�е�Fe2+�γ���Һ����Fe2+����ΪFe3+��ʹFe3+ת��Ϊ����������������ת��Ϊ��������ͨ���ⶨ�����������������㲹Ѫ������Ԫ�صĺ�����
��1��Fe3+��KSCN��Һ�Ժ�ɫ�����������ڼ���Fe3+���ڣ����Լ�����������Fe2+����ΪFe3+������Fe2+�����ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ��˫��ˮ����Һ��ΪѪ��ɫ��˵������Fe2+��
��2��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ��
��3��������ǽ�Fe3+ת��Ϊ��������������
��4���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
��5��������Ԫ���غ��֪ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ���������������ݴ˼��㣮
��6���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ��ҩ�ס��������ձ�����ͷ�ιܡ�250mL����ƿ��
�ڼ������ܾ���ǿ�����ԣ����ܱ����Ը��������������ֹӰ�����Ը��������Һ�������Ӱ��ⶨ�����
�۸������Ϊ��ɫ�����ζ����յ�ʱ��Fe2+����ȫ��������������һ�θ�����ز���Ӧ����Һ����ɫΪ��ɫ��
��7��������Ԫ���غ��֪16.8mg����ΪFeSO4•7H2OƬ�����������������ݻ�ѧʽ����Ԫ�����������ļ���������FeSO4•7H2O��Ƭ����������
��� �⣺��1��Fe3+��KSCN��Һ�Ժ�ɫ�����������ڼ���Fe3+���ڣ����Լ�����������Fe2+����ΪFe3+������Fe2+�����ȵμ�KSCN��Һ����Һ����ɫ���ٵμ���ˮ��˫��ˮ����Һ��ΪѪ��ɫ��˵������Fe2+��
�ʴ�Ϊ����ˮ����Һ��dz��ɫ��ΪѪ��ɫ��
��2��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ����Ӧ���ӷ���ʽΪ2Fe2++H2O2+2H+�T2Fe3++2H2O��
�ʴ�Ϊ�������������ܽ�Fe2+ȫ������ΪFe3+��ͬʱ����ˮ��������п�����ʣ�
��3��������ǽ�Fe3+ת��Ϊ����������������Ӧ���ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
�ʴ�Ϊ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��
��4���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
�ʴ�Ϊ��ϴ�ӣ���ȴ��
��5��ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ������������������ÿƬ��Ѫ������Ԫ�ص�����$\frac{ag��\frac{112}{160}}{10}$=0.07ag���ʴ�Ϊ��0.07a��
��6���پ�ȷ����һ�����ʵ���Ũ�ȵ�KMnO4��Һ250mL������ʱ��Ҫ����������ƽ��ҩ�ס��������ձ�����ͷ�ιܡ�250mL����ƿ��
�ʴ�Ϊ��250mL����ƿ��
�ڸ��������ǿ�����ԣ��ܽ�����������������������ԣ�������������������ֻ����ϡ�����ữ���ʴ�Ϊ��b��
�۸������Ϊ��ɫ�����ζ����յ�ʱ��Fe2+����ȫ��������������һ�θ�����ز���Ӧ����Һ����ɫΪ��ɫ���ʴ�Ϊ���ϣ�
��7��14mg����ΪFeSO4•7H2OƬ��������������������ҪFeSO4•7H2OƬ������Ϊ��$\frac{14}{56}��278$ ��mg=69.5mg��
�ʴ�Ϊ��69.5��
���� ���⿼��ѧ����ʵ��ԭ����ʵ����������⡢���ʷ����ᴿ��Ԫ�ػ��������ʡ�������ԭ�ζ�Ӧ�á���ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

| A�� | �٢ڢ� | B�� | �ڢۢ� | C�� | �ۢ٢� | D�� | �ڢ٢� |
| A�� | 0.5 L1mol/L NaHS��Һ�У�N��Na+��+N��HS-��=NA | |
| B�� | 12.4g���ף�����ʽΪP4���к���P-P���ۼ�0.6NA | |
| C�� | �����£�pH=2�Ĵ�����Һ�������е�H+��Ϊ0.01NA | |
| D�� | ��92gN2O4������������У��ָ������³�ѹʱ���������������ΪNA |
| A�� | ����Ԫ���У�Ԫ��Z�ķǽ�������ǿ | |
| B�� | X��Y��Z����Ԫ�ز������γ����ӻ����� | |
| C�� | Y��Z��W����Ԫ�ص�ԭ�Ӱ뾶��С˳��r��W����r��Z����r��Y�� | |
| D�� | YW3��ZW2��Z2W2�и�ԭ���������ﵽ8�����ȶ��ṹ |
| A�� | ���з�Һ����ʱ��Ӧ�ȴ��Ͽڻ�����ʹ���ϵİ��۶�©���ڵ�С�ף�Ȼ����������²�Һ����¿ڷų����ϲ�Һ���©���Ͽڵ��� | |
| B�� | ֽ������������ֽΪ����֧���ˮΪ�̶��࣬�л��ܼ��������࣮�ø÷����ɷ���Fe3+��Cu2+��������Ѭ��ɫ����ֽ�Ϸ����ֺ���ɫ�ߵ� | |
| C�� | ��ʽ�ζ��ܡ���ʽ�ζ��ܡ�����ƿ����Һ�ܶ���ȷ��ȡһ�����Һ���������������ʹ��ʱ��Ҫ���м���Ƿ�©ˮ��ˮϴ����ϴ��עҺ������Һ��ȼ������� | |
| D�� | ���Ʊ�����ؾ����ʵ���У����ȹ���ʱ���н���Һ��С�ձ���Ԥ�ȼ�2mL����ˮ���Է�����ʱ�Ȼ��ƾ������� |
| A�� | �Ȼ��� | B�� | ���� | C�� | ��Ȳ | D�� | ���� |
| A�� | c��N2����c��H2����c��NH3��=1��3��2 | |
| B�� | N2��H2��NH3�������������ٸı� | |
| C�� | N2��H2�����ʵ���֮����NH3�����ʵ�����2�� | |
| D�� | ��λʱ����ÿ����1 mol N2��ͬʱ����2 mol NH3 |
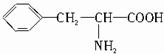 ��B�Ľṹ��ʽΪ
��B�Ľṹ��ʽΪ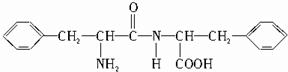 ��
��