��Ŀ����
�����16�֣���ʯ����Ҫ�ɷ���Ca5F(PO4)3��������MgO��Fe2O3�����ʡ���ҵ������ʯΪԭ���Ʊ�H3PO4�ij����������£�

��֪��Ca5F(PO4)3+ 7H3PO4��5Ca (H2PO4)2+HF
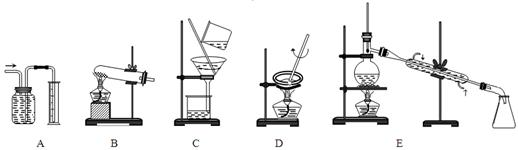
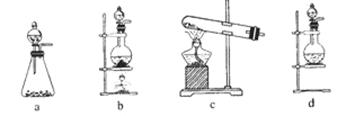
��1���������ַ�����ʵ�����ܽ���ʯ________����ܡ����ܡ����ò���������ԭ����_____________________________________��
��2���������������___________
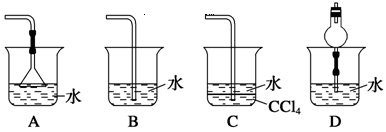
��3����ʵ������ʵ�ֲ�����͢�����Ҫ�IJ���������_______________________���Ʋ����ȡ��һ�����е�������_________��
a. ����ȡ����ˮ�������� b. ��ͬ�����£�����ȡ�����ܶȱ�ˮС
c. �����ڸ���ȡ���е��ܽ�Ⱥ�С d. ijЩ����������ڸ���ȡ���е��ܽ�Ⱥ�С
��4�����ø����̳���ȡ�����⣬����__________________�ȸ���Ʒ��������˵������һ�ָ���Ʒ����;____________________________________��
��5����ֱ���������ܽ���ʯ�Ĺ�����ȣ��ù��յ��ŵ���____________________��

��֪��Ca5F(PO4)3+ 7H3PO4��5Ca (H2PO4)2+HF
��1���������ַ�����ʵ�����ܽ���ʯ________����ܡ����ܡ����ò���������ԭ����_____________________________________��
��2���������������___________
��3����ʵ������ʵ�ֲ�����͢�����Ҫ�IJ���������_______________________���Ʋ����ȡ��һ�����е�������_________��
a. ����ȡ����ˮ�������� b. ��ͬ�����£�����ȡ�����ܶȱ�ˮС
c. �����ڸ���ȡ���е��ܽ�Ⱥ�С d. ijЩ����������ڸ���ȡ���е��ܽ�Ⱥ�С
��4�����ø����̳���ȡ�����⣬����__________________�ȸ���Ʒ��������˵������һ�ָ���Ʒ����;____________________________________��
��5����ֱ���������ܽ���ʯ�Ĺ�����ȣ��ù��յ��ŵ���____________________��
�����16�֣�(1)���ܣ�HF�ḯʴ���� (2)���ˣ�
(3)��Һ©�����ձ��� a��d����1�֣���2�֣�
(4)�����⡢ʯ�ࣻ����;�ԣ�ֻҪ����������������ʯ�����һ��;���ɣ�
(5)��ʯ�����ܽ�
(3)��Һ©�����ձ��� a��d����1�֣���2�֣�
(4)�����⡢ʯ�ࣻ����;�ԣ�ֻҪ����������������ʯ�����һ��;���ɣ�
(5)��ʯ�����ܽ�
��1������HF�ḯʴ�����ܸ�ʴ���������Բ����ò���������
��2������Һ�з��������ķ����ǹ��ˣ����Եõ�ʯ��ķ����ǹ��ˡ�
��3����ȡ����Ҫ���������Ƿ�Һ©�����ձ�����ȡ��Ӧ�����������Ǻ�ԭ�ܼ������ܣ��ұ���ȡ�������ڸ���ȡ���ߵ��ܽ�ȴ���ԭ�ܼ��е��ܽ�ȣ����Դ�ѡad��
��4����������ͼ��֪�����ø���Ʒ�Ƿ������ʯ�ࡣ����������ڵ�̲�����ʯ������ڹ�ҵ�����ȡ�
��5����������ͼ��ԭ����֪���ù��յ��ŵ�����ʯ�����ܽ⡣
��2������Һ�з��������ķ����ǹ��ˣ����Եõ�ʯ��ķ����ǹ��ˡ�
��3����ȡ����Ҫ���������Ƿ�Һ©�����ձ�����ȡ��Ӧ�����������Ǻ�ԭ�ܼ������ܣ��ұ���ȡ�������ڸ���ȡ���ߵ��ܽ�ȴ���ԭ�ܼ��е��ܽ�ȣ����Դ�ѡad��
��4����������ͼ��֪�����ø���Ʒ�Ƿ������ʯ�ࡣ����������ڵ�̲�����ʯ������ڹ�ҵ�����ȡ�
��5����������ͼ��ԭ����֪���ù��յ��ŵ�����ʯ�����ܽ⡣

��ϰ��ϵ�д�
�����Ŀ
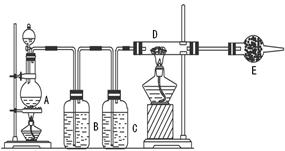
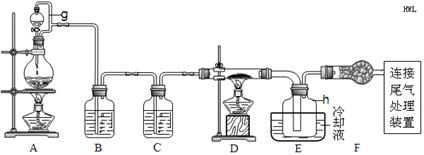
 2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣
2CaSO4+2Cl2��+2H2O �����������������ȡ��������֤�����ʵ�ʵ�顣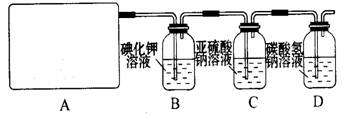
 5Fe3����Mn2����4H2O
5Fe3����Mn2����4H2O