��Ŀ����
��14�֣�ʵ���ǽ��л�ѧ�о�����Ҫ�ֶ�֮һ��������������⡣
��1������A��E����ѧ��ѧʵ���г����ļ���ʵ��װ�á�
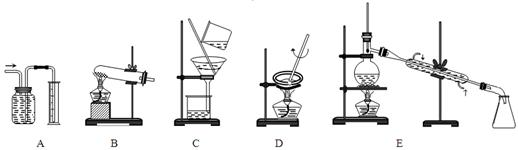
��Ϊ��������ʵ����ѡ���ʵ�װ�ã��������ĸ��
������װ���� ���ڹ���װ���� ��
������װ���� ����I2��CCl4��Һ����ȡI2ѡ�� ��
��2������ʵ�����������ȷ����________
a����ֽ�����Թ���װ��ĩ״ҩƷʱ���Թ�Ӧ�Ⱥ����ֱ��
b����ȡ�������ƹ���ʱ��Ӧ���������ƹ���ֱ�ӷ�����������ڣ��ұ����̷�����
c���Թܡ��ձ�����Ͳ������ƿ�������þƾ���ֱ�Ӽ���
d������ֽ������������ʱ����������ֽ��ˮ��ϴ������۲���ֽ��ɫ�ı仯
��3��ʵ������Ҫ450 mL 0.1 mol/LNaOH��Һ��500 mL 0.5 mol/L������Һ����ش��������⡣
������ͼ��ʾ�����У�����������Һ�϶�����Ҫ���� ��������ţ���ͼ�����������⣬����������Һ����Ҫ�IJ��������� ��
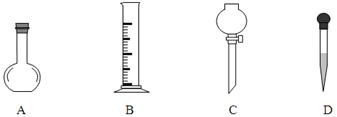
������450 mL 0.1 mol/L NaOH��Һ��ʵ�鲽�����£�
a������Ӧ��ȡ�������ƹ��������Ϊ g��
b�������������ƹ��塣
c�����ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲע������ƿ��
d��������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e���Ǻ�ƿ�����������µߵ���ҡ�ȡ�
f������������ƿ�м�����ˮ���̶�����1��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������С�
�����������ȷ����˳��Ϊ ��������ţ�
��1������A��E����ѧ��ѧʵ���г����ļ���ʵ��װ�á�

��Ϊ��������ʵ����ѡ���ʵ�װ�ã��������ĸ��
������װ���� ���ڹ���װ���� ��
������װ���� ����I2��CCl4��Һ����ȡI2ѡ�� ��
��2������ʵ�����������ȷ����________
a����ֽ�����Թ���װ��ĩ״ҩƷʱ���Թ�Ӧ�Ⱥ����ֱ��
b����ȡ�������ƹ���ʱ��Ӧ���������ƹ���ֱ�ӷ�����������ڣ��ұ����̷�����
c���Թܡ��ձ�����Ͳ������ƿ�������þƾ���ֱ�Ӽ���
d������ֽ������������ʱ����������ֽ��ˮ��ϴ������۲���ֽ��ɫ�ı仯
��3��ʵ������Ҫ450 mL 0.1 mol/LNaOH��Һ��500 mL 0.5 mol/L������Һ����ش��������⡣
������ͼ��ʾ�����У�����������Һ�϶�����Ҫ���� ��������ţ���ͼ�����������⣬����������Һ����Ҫ�IJ��������� ��

������450 mL 0.1 mol/L NaOH��Һ��ʵ�鲽�����£�
a������Ӧ��ȡ�������ƹ��������Ϊ g��
b�������������ƹ��塣
c�����ձ��е���Һע������ƿ��������������ˮϴ���ձ��ڱ�2��3�Σ�ϴ��ҺҲע������ƿ��
d��������������ˮ�ܽ�����õ��������ƹ��壬��ȴ��
e���Ǻ�ƿ�����������µߵ���ҡ�ȡ�
f������������ƿ�м�����ˮ���̶�����1��2 cmʱ�����ý�ͷ�ιܵμ�����ˮ����Һ����̶������С�
�����������ȷ����˳��Ϊ ��������ţ�
��14�֣�
��1��A��1�֣� C��1�֣� D��1�֣� E��1�֣�
��2��bcd��2�֣�
��3����AC��2�֣� �ձ�����������500 mL����ƿ��2�֣�ÿ��һ�ֿ�1�֣�
��2.0��2�֣� abdcfe��2�֣�
��1��A��1�֣� C��1�֣� D��1�֣� E��1�֣�
��2��bcd��2�֣�
��3����AC��2�֣� �ձ�����������500 mL����ƿ��2�֣�ÿ��һ�ֿ�1�֣�
��2.0��2�֣� abdcfe��2�֣�
���������
��1��Aװ������ˮ����װ�ã�Bװ���ǹ�����ȷ�Ӧװ�ã�Cװ���ǹ���װ�ã�Dװ��������Һ��װ�ã�Eװ��������װ�á�
��2����Ϊ�������ƾ��и�ʴ�Ժ��׳������ʣ�����ʱӦ�÷���С�ձ���������г�������Ͳ���ܼ��ȡ��Թܿ���ֱ�Ӽ��ȣ���ֽӦ�������Ӽ�ס�������壬��������ֱ��ȥ����ֽ��
��3�������������ƺ�������Ҫ�õ�������ƽ����Ͳ��500 mL����ƿ����ֽ���ձ�������������ͷ�ιܵ�����������Ҫƽ����ƿ�ͷ�Һ©������ȱ���ձ�����������500 mL����ƿ������
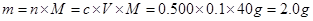 ��������450mL����������㣬��Ϊû������ݻ�������ƿ��������Һ���ƵIJ��裺һ�㣨���㣩�����ƣ������������ܣ��ܽ⣩����ת��ת�ƣ�����ϴ��ϴ�ӣ������������ݣ�����ҡ��ҡ�ȣ�����װ��װƿ��������������ǩ����Ӧ����abdcfe��
��������450mL����������㣬��Ϊû������ݻ�������ƿ��������Һ���ƵIJ��裺һ�㣨���㣩�����ƣ������������ܣ��ܽ⣩����ת��ת�ƣ�����ϴ��ϴ�ӣ������������ݣ�����ҡ��ҡ�ȣ�����װ��װƿ��������������ǩ����Ӧ����abdcfe��������ʵ����ѧ��������ʧ�ֵ㣬��Ȼ�������ڻ����⣬������ѧ����Ȼ���ѵ㡣

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
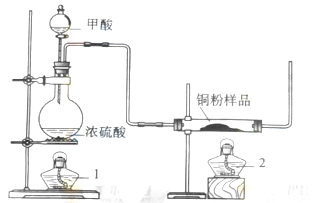 .��1���Ʊ�һ����̼�Ļ�ѧ����ʽ�� ��
.��1���Ʊ�һ����̼�Ļ�ѧ����ʽ�� ��
