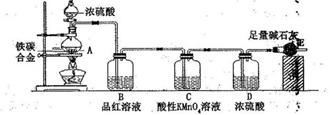
��Ŀ����
Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飬��ش��������⡣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1mol/L FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��ݡ� | �Թ�A�в��ٲ������ݣ� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ����� |
��1����������ֽ�Ļ�ѧ����ʽΪ_____________________________________��
��2��ʵ��ٵ�Ŀ����_________________________________________________��
ʵ���еμ�FeCl3��Һ��Ŀ����_______________________________________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_______________________����ʵ�������ṩ�ļ����Լ�����
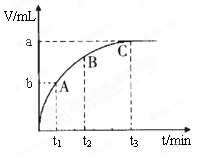
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ����A��B��C��������ʾ��˲ʱ��Ӧ������������______��
��1��2H2O2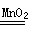 2H2O+O2��
2H2O+O2��
��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�� �ӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲�
��3������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/LFeCl3��Һ���۲�������ݵ�����
��4��C��
���������������1���ڴ����������£���������ֽ�����������ˮ����Ӧ�Ļ�ѧ����ʽ��2H2O2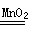 2H2O+O2����
2H2O+O2����
��2���ֱ����Թ�A��B�м��� 5mL 5% H2O2��Һ��������1��2 ��1mol/L FeCl3��Һ�����Թ��о����������ݳ��֣�˵����������ֽ��ܷ������Թ�A��B�о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��У���֧�Թܲ�ͬ�����Թ�A���¶ȱ��Թ�B���¶ȵͣ�˵���о������¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʼ�ӵμ�FeCl3��Һ��Ŀ�ļӿ�H2O2�ֽ⡣���ʵ��ٵ�Ŀ�����о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죻�������Ȼ����������Ǽӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲졣
��3��Ӱ�컯ѧ��Ӧ���ʵ����������Ũ�ȡ��¶ȡ������ѹǿ������������ı�����ȡ����Ϊ�ӿ췴Ӧ���ʣ��ɴ��¶ȡ��������Ӱ��Ƕȿ��ǣ�������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/L FeCl3��Һ���۲�������ݵ����ʣ�
��4����ͼ��������ʾʱ�䣬�������ʾ��������������ʱ��Խ�����ɵ�����Խ�࣬��Ӧ����Խ�죬���ߵ�б��Խ����������������ΪC��
���㣺������������Է�Ӧ����Ӱ���ʵ��̽��
�����������Ǹ߿��еij������ͣ����ڻ���������Ŀ��飬�ѶȲ����ʱ��ע����ȷʵ���ԭ��������Ӱ�췴Ӧ���ʵ����������һ���з������ɣ�����������ѧ������˼ά�����淶�Ͻ���ʵ�����������

������ʵ���������ȷ����
| A����Һʱ����Һ©���²�Һ����¶˷ų����ϲ�Һ����Ͽڵ��� |
| B������ʱ��Ӧʹ�¶ȼ�ˮ����������ƿ֧�ܿ� |
| C�������ᾧʱӦ����Һֱ������ |
| D�������Ǹ�ʴ��ҩƷӦ����������ƽ���̵ij���ֽ�ϣ��������������ƽ���̵ij���ֽ�� |
| ʵ�鲽�� | ���� | ���� |
| �ٷֱ�ȡ�����2mol/L���������Թ��� �� | Mg�����������ʿ���Fe��Cu�����Ա仯 | ��������Խ���ã���Ӧ����Խ�� |
��2����ͬѧ��ʵ��Ŀ����̽�� ��
��ͬѧΪ�˸���ȷ���о�Ũ�ȶԷ�Ӧ���ʵ�Ӱ�죬������ͼ��ʾװ�ý��ж���ʵ�顣

��3����ͬѧ��ʵ����Ӧ�òⶨ�������� ��
��4����ͬѧ���ʵ��Ӧѡ�õ�ʵ��ҩƷ�� ��
��ѧ������������������أ�����˵����ȷ����
| A����ʳ�ؽ��������������ж����ɺȴ�����ʳ��ˮ�ⶾ |
| B�����ʵ���֬�����ŵ�������ζ����������֬������ˮ�ⷴӦ |
| C�����ݹ����������Һ�Ĺ���������ˮ��������Ϊ���ӳ�ˮ�������� |
| D����װú̿ȼ�չ��̵ġ�����װ�ã���Ҫ��Ϊ�����ú�������� |
����˵������ȷ����
| A��̼���ơ�̼��������Һ���Լ��ԣ��Ҷ�����Ϊʳ�ü� |
| B�������ڵĵ����ʲ��Ϸֽ⣬��������ˮ�Ͷ�����̼�ų����� |
| C���ҹ�����ͳ��ʳ������Ϊ�������ۡ���ά�ض����������� |
| D�����������ķ�ʽ֬����Խ�������������Һֲ̬���ͼ���ʱ������ |