��Ŀ����
12��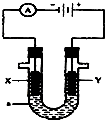 ��ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺
��ͼ��ʾһ�����أ�װ�е��Һa��X��Y������缫�壬ͨ��������ֱ����Դ��������ش��������⣺��1����X��Y���Ƕ��Ե缫��a��NaOH��Һ��
�ٵ�����X���ϵĵ缫��ӦʽΪ2H++2e-=H2��
��Y�缫�ϵĵ缫��ӦʽΪ4OH--4e-=2H2O+O2��
���ܵĵ�⻯ѧ����ʽΪ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2��
�ܵ�����ҺPH�ı仯Ϊ��������С��
��2����X��Y���Ƕ��Ե缫��a�� H2SO4����Һ��
�ٵ�����X���ϵĵ缫��ӦʽΪ2H++2e-=H2��
��Y�缫�ϵĵ缫��ӦʽΪ4OH--4e-=2H2O+O2��
���ܵĵ�⻯ѧ����ʽΪ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2��
�ܵ�����ҺPH�ı仯Ϊ��С�������� ��С��
��3����X��Y���Ƕ��Ե缫��a ��Na2SO4����Һ��
�ٵ�����X���ϵĵ缫��ӦʽΪ2H++2e-=H2��
��Y�缫�ϵĵ缫��ӦʽΪ4OH--4e-=2H2O+O2��
���ܵĵ�⻯ѧ����ʽΪ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2��
�ܵ�����ҺPH�ı仯Ϊ���䣨�������С��
��4����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ����˵�������ʷ����ĵ缫��Ӧ����д����
��X�缫�IJ����Ǿ�ͭ���缫��ӦʽΪCu-2e-=Cu2+��
��Y�缫�IJ����Ǵ�ͭ���缫��ӦʽΪCu2++2e-=Cu��
���� ��1����X��Y���Ƕ��Ե缫��a��NaOH��Һ��Y�缫�����������ӷŵ�����������X�缫�������ӷŵ磬�൱�ڵ��ˮ��
��2����X��Y���Ƕ��Ե缫��a��H2SO4����Һ��Y�缫�����������ӷŵ�����������X�缫�������ӷŵ磬�൱�ڵ��ˮ��
��3����X��Y���Ƕ��Ե缫��a ��Na2SO4����Һ��Y�缫�����������ӷŵ�����������X�缫�������ӷŵ磬�൱�ڵ��ˮ��
��4����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������Ӧ���Ǵ�ͭ�������Ǵ�ͭ����X�缫�Ǵ�ͭ��Y�缫�Ǵ�ͭ��������ͭ���ӷŵ磮
��� �⣺��1����X��Y���Ƕ��Ե缫��a��NaOH��Һ��������������Y��������������������ʧ���ӣ���ӦʽΪ��4OH--4e-=2H2O+O2�����븺��������X���������������ӵõ��ӣ���ӦʽΪ��2H++2e-=H2������⻯ѧ����ʽΪ���ˮ����ӦʽΪ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�������Ե�����ҺŨ����������ǿ����pH���
�ʴ�Ϊ��2H++2e-=H2����4OH--4e-=2H2O+O2����2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�������
��2����X��Y���Ƕ��Ե缫��a��H2SO4����Һ��������������Y��������������������ʧ���ӣ���ӦʽΪ��4OH--4e-=2H2O+O2�����븺��������X���������������ӵõ��ӣ���ӦʽΪ��2H++2e-=H2������⻯ѧ����ʽΪ���ˮ����ӦʽΪ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�������Ե�����ҺŨ������������ǿ����pH��С��
�ʴ�Ϊ��2H++2e-=H2����4OH--4e-=2H2O+O2����2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2������С��
��3����X��Y���Ƕ��Ե缫��a ��Na2SO4����Һ��������������Y��������������������ʧ���ӣ���ӦʽΪ��4OH--4e-=2H2O+O2�����븺��������X���������������ӵõ��ӣ���ӦʽΪ��2H++2e-=H2������⻯ѧ����ʽΪ���ˮ����ӦʽΪ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2������Ȼ����Na2SO4��ҺŨ��������ȻΪ���ԣ���pH���䣬
�ʴ�Ϊ��2H++2e-=H2����4OH--4e-=2H2O+O2����2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2�������䣻
��4����Ҫ�õ�ⷽ��������ͭ�����Һaѡ��CuSO4��Һ������Ӧ���Ǵ�ͭ�������Ǵ�ͭ����X�缫�Ǵ�ͭ��Y�缫�Ǵ�ͭ������������ӦΪ��Cu-2e-=Cu2+��������ͭ���ӷŵ磬�缫��ӦʽΪCu2++2e-=Cu��
�ʴ�Ϊ����ͭ��Cu-2e-=Cu2+����ͭ��Cu2++2e-=Cu��
���� ���⿼��ԭ���ԭ����Ϊ��Ƶ���㣬������ѧ���ķ��������Ŀ��飬�����漰�缫��Ӧʽ����д�������֪ʶ�㣬֪�����ӷŵ�˳�缫��Ӧʽ����д��������Ŀ�ѶȲ���

 ������ϰ�ο����뵥Ԫ���ϵ�д�
������ϰ�ο����뵥Ԫ���ϵ�д� �����Ծ���ĩ���100��ϵ�д�
�����Ծ���ĩ���100��ϵ�д� ˫��ͬ������ѵ��ϵ�д�
˫��ͬ������ѵ��ϵ�д�| A�� | ��״���£�2.24LSO3���еķ���������0.1NA | |
| B�� | 1 mol Na2O2������������Ϊ2NA | |
| C�� | ���³�ѹ�£�16gO2 ��O3�Ļ�����庬�е���ԭ����ΪNA | |
| D�� | ���³�ѹ���������£�33.6 LCl2��3.0g H2��Ӧ�����ɵ�HCl������ĿΪ3NA |
| A�� | HCI��Һ�����Ա�H2S��Һ������ǿ | |
| B�� | ��������������Һ��Ӧ��H2S+C12�T2HCl+S�� | |
| C�� | �Ȼ���������ȶ� | |
| D�� | HClO4�����Ա�H2SO4ǿ |
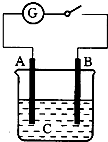 ��ͼ��ʾ����A��B����C����Һ�У��պϵ�����������ָ��ᷢ��ƫת����B�������ݲ�������A��B��C������������һ�����ʣ�������
��ͼ��ʾ����A��B����C����Һ�У��պϵ�����������ָ��ᷢ��ƫת����B�������ݲ�������A��B��C������������һ�����ʣ�������| A�� | A-Cu����B-Zn����C-CuSO4 | B�� | A-Ag����B-Fe����C-HCl | ||
| C�� | A-Zn����B-ʯī��C-H2SO4 | D�� | A-ʯī��B-Zn����C-H2SO4 |
| A�� | A��B��C��D | B�� | D��A��B=C | C�� | A=B��C=D | D�� | D��A��B��C |
| A�� | �к��ɫ���� | B�� | �к��ɫ������������� | ||
| C�� | ������������������� | D�� | ��������������� |
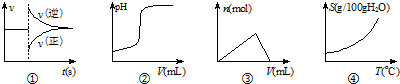
| A�� | ����ͼ�ٱ�ʾ��ij��ѧƽ����ϵ�ı��¶Ⱥ�Ӧ������ʱ��ı仯 | |
| B�� | ����ͼ�ڱ�ʾ��һ������������Һ�еμ�һ��Ũ�ȵ�����������ҺʱpH�ı仯 | |
| C�� | ����ͼ�۱�ʾ��һ������NaAlO2��Һ�еμ�һ��Ũ�ȵ�HCl��Һʱ�IJ������������ʵ����仯 | |
| D�� | ����ͼ�ܿ��Ա�ʾ���еĹ��������ܽ�����¶ȵı仯 |
| A�� | ��ԭ�����������ӷ�Ӧ�����Ȼ��ƺ���ṹ���ȶ�����ǿ | |
| B�� | ѡ��ȼ��ֻҪ����ȼ��ȼ��ֵ�Ĵ�С | |
| C�� | ʧ�����ѵ�ԭ�ӻ�õ��ӵ�����һ��ǿ | |
| D�� | ����ԭ��֮�������ýл�ѧ�� |