��Ŀ����
����Ŀ����Ҫ����д��
(1) �ҹ��Ŵ��Ĵ���֮һ�ĺڻ�ҩ������Ƿۡ�����غ�ľ̿����һ��������϶��ɵģ���ըʱ�Ļ�ѧ��ӦΪ��S+2KNO3+3C=K2S+N2��+3CO2�����÷�Ӧ����������_____����ԭ����______��_____Ԫ�ر�������ÿ����6.72LCO2(��״����)������Ҫ__g��μӷ�Ӧ������˫���ŷ�����ʾ����ת�Ƶķ������Ŀ��______��
(2)�����������ʹ�õĽ���֮һ��
�ټ���![]() �����
�����![]() ��Һ�ij��÷�����_____�����߱���������_____ ��
��Һ�ij��÷�����_____�����߱���������_____ ��
�ڵ��ӹ�ҵ����![]() ��Һ��ʴ���ھ�Ե���ϵ�ͭ�����Ȼ��������Ȼ�ͭ������ӡˢ��·�壬��д��
��Һ��ʴ���ھ�Ե���ϵ�ͭ�����Ȼ��������Ȼ�ͭ������ӡˢ��·�壬��д��![]() ��Һ��ͭ��Ӧ�����ӷ���ʽ______��
��Һ��ͭ��Ӧ�����ӷ���ʽ______��
��![]() �����Ʊ�ʵ�飺
�����Ʊ�ʵ�飺
ʵ�鲽�裺ȡһ��С�ձ�������25mL����ˮ�����ձ��е�����ˮ���������ڣ����ˮ����μ���5��6��FeCl3������Һ��������С�
ʵ�������ձ�����Һ��_____ɫ����ѧ����ʽ��_____��
���𰸡�S��KNO3 C C 3.2  �����ЧӦ ��ɢ������ֱ����С 2Fe3++Cu==2Fe2++2Cu2+ ���ɫ FeCl3��3H2O =Fe(OH)3�����壩��3HCl
�����ЧӦ ��ɢ������ֱ����С 2Fe3++Cu==2Fe2++2Cu2+ ���ɫ FeCl3��3H2O =Fe(OH)3�����壩��3HCl
��������
��1����ӦS+2KNO3+3C�TK2S+N2��+3CO2���У�N��SԪ�ػ��ϼ۽��ͣ�����ԭ��CԪ�ػ��ϼ����ߣ����������ݴ˷�����
��2��Fe(OH)3������Ʊ��������ڷ�ˮ�еμӱ����Ȼ�����Һ����������Һ�ʺ��ɫ�����ֽ������Һ�ķ������ö����ЧӦ���������������ɢϵ�ı��������Ƿ�ɢ�ʵĿ�����С��
��1����������������N��SԪ�ػ��ϼ۽��ͣ���������S��KNO3��CԪ�ػ��ϼ����ߣ�����������ԭ����C�����ɱ�״����6.72LCO2����ʱ����0.3mol������0.1molS�μӷ�Ӧ������Ϊ3.2g��˫���ŷ���ʾ����ת�Ƶķ������Ŀ�� ���ʴ�Ϊ��S��KNO3����C��C��3.2��
���ʴ�Ϊ��S��KNO3����C��C��3.2�� ��
��
��2���ټ��������Һ�ij��÷����Ƕ����ЧӦ����ɢ����ֱ���Ĵ�С�ǽ����������ɢϵ�ı������𣬹ʴ�Ϊ�������ЧӦ����ɢ������ֱ����С��
��FeCl3��Һ��ͭ��Ӧ�����ӷ���ʽΪ2Fe3++Cu�T2Fe2++Cu2+���ʴ�Ϊ��2Fe3++Cu�T2Fe2++Cu2+��
��Fe(OH)3������Ʊ��������ڷ�ˮ�еμӱ����Ȼ�����Һ����������Һ�ʺ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��FeCl3��3H2O =Fe(OH)3(����)��3HCl���ʴ�Ϊ�����ɫ��FeCl3��3H2O =Fe(OH)3(����)��3HCl��

����Ŀ��H2O2����ȡ��������ˮ���������Ӧ���ǵ�ǰ��ѧ�о����ȵ㡣 �ش��������⣺
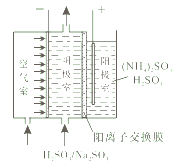
��1��������ͬ��������������������[��NH4��2S2O8]��ԭ����ͼ��ʾ����������������Ӧ��������_______�������ĵ缫��ӦʽΪ_________��
��2��100��ʱ���ڲ�ͬ�������Ӵ����£�����������24h�ķֽ��ʼ��±���
���� | ������/��mg��L-1�� | �ֽ���/% | ���� | ������/��mg��L-1�� | �ֽ���/% | |
�� | �� | 2 | Fe3+ | 1.0 | 15 | |
Al3+ | 10 | 2 | Cu2+ | 0.1 | 86 | |
Zn2+ | 10 | 10 | Cr3+ | 0.1 | 96 |
���ϱ����ݿ�֪����ʹ��������ֽⷴӦ��ܽ�������������_______�����˹�������ʱ����ѡ�õ���������Ϊ________�����ţ���
A ���� B ��ͭ C ���� D �����
��3���������������£�H2O2��һ�ִ��ֽ�������£�
H2O2��aq����Mn2+��aq��=OH��aq����Mn3+��aq����OH����aq�� ��H��a kJ/mol
H2O2��aq����Mn3+��aq����2OH����aq��=Mn2+��aq������O2- ��aq����2H2O��l�� ����b kJ/mol
OH��aq������O2-��aq��=O2��g����OH����aq�� ��H��c kJ/mol
��2H2O2��aq��=2H2O��l����O2��g������H��_________���÷�Ӧ�Ĵ���Ϊ________��
��4��298 Kʱ����10 mL a mol��L1 NaH2PO2��10 mL 2a mol��L1 H2O2��Һ��10 mL NaOH��Һ��ϣ�������Ӧ��H2PO2-��aq����2H2O2��aq����2OH��aq��![]() PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
PO43-��aq����4H2O��l������Һ��c��PO43-���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
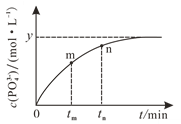
�����п��жϷ�Ӧ�ﵽƽ�����_______�����ţ���
a c��H2PO2-����y mol��L1
b ��Һ��pH���ٱ仯
c v��H2O2����2v��H2PO2-��
d c��PO43-��/c��H2PO2-�����ٱ仯
��tmʱv��_____tnʱv����������������С������������������
����ƽ��ʱ��Һ��pH��12����÷�Ӧ��ƽ�ⳣ��KΪ___________��