��Ŀ����
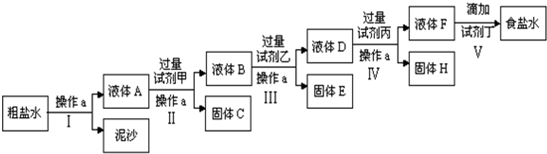
2����Ҫ��д������ʽ����1��д���������ʵĵ��뷽��ʽ��
��̼�����ƣ�NaHCO3�THCO3-+H+
�ڴ��NH3•H2O?NH4++OH-
��2��д�����з�Ӧ���Ȼ�ѧ����ʽ��
��������������Ӧ����1molˮ��������241.8kJ��H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8kJ/mol
�����Ƿ���ʱ�����£�N2H4����ȼ�ϣ�1mol N2H4��l����O2��g����ȼ�գ�����N2��g����H2O��l�����ų�622kJ������N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ•mol-l
��3��д����������ˮ������ӷ���ʽ��
��K2CO3��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-
�ڣ�NH4����2SO4��NH4++H2O?NH3•H2O+H+
��FeCl3��Fe3++3H2O?Fe��OH��3+3H+��
���� ��1����̼����������ǿ����ʣ���ȫ����������Ӻ�̼��������ӣ�̼��������Ӳ��ܲ���һˮ�ϰ�Ϊ������ʣ����ֵ����笠����Ӻ����������ӣ�
��2���ٸ����Ȼ�ѧ����ʽ����дԭ��д������ȼ��������̬ˮ���Ȼ�ѧ����ʽ�������е������ͻ�ѧ������Ҫ��Ӧ���ڷ���ʱ�ʱ�ֵΪ��ֵ�������Ȼ�ѧ����ʽ����д�������
��3����̼���Ƕ�Ԫ���ᣬ����̼������ӷ�����ˮ�⣬��һ��̼�������ˮ������̼��������ӣ��ڶ���̼���������ˮ������̼������ӣ�
�������Ϊǿ�������Σ�笠�����ˮ������һˮ�ϰ��������ӣ�
���Ȼ���Ϊǿ�������Σ�������ˮ���������������������ӣ�
��� �⣺��1��̼����������ǿ����ʣ���ȫ���룬̼����������Ƕ�Ԫ����̼�����ʽ�ε�������ӣ�������ȫ���룬���ܲ�д�����뷽��ʽΪ��NaHCO3�THCO3-+H+��
�ʴ�Ϊ��NaHCO3�THCO3-+H+��
��NH3•H2OΪһԪ������ֵ��룬���뷽��ʽΪ��NH3•H2O?NH4++OH-��
�ʴ�Ϊ��NH3•H2O?NH4++OH-��
��2��������������Ӧ����1molˮ��������241.8kJ���÷�Ӧ���Ȼ�ѧ����ʽΪ��H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8kJ/mol��
�ʴ�Ϊ��H2��g��+$\frac{1}{2}$O2��g��=H2O��g����H=-241.8kJ/mol��
��1mol N2H4��l����O2��g����ȼ�գ�����N2��g����H2O��l�����ų�622kJ��������ʱ�ʱ�ֵΪ��ֵ�����Ը÷�Ӧ���Ȼ�ѧ����ʽΪN2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ•mol-l��
�ʴ�Ϊ��N2H4��g��+O2��g��=N2��g��+2H2O��l����H=-622kJ•mol-l��
��3����̼���Ƕ�Ԫ���ᣬ����̼������ӷ�����ˮ�⣬��һ��̼�������ˮ������̼��������ӣ��ڶ���̼���������ˮ������̼������ӣ�������ˮ�ⷽ��ʽ�ֱ�Ϊ��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-��
�ʴ�Ϊ��CO32-+H2O?HCO3-+OH-��HCO3-+H2O?H2CO3+OH-��
��笠�����ˮ������һˮ�ϰ��������ӣ�ˮ�����ӷ�ӦΪNH4++H2O?NH3•H2O+H+��
�ʴ�Ϊ��NH4++H2O?NH3•H2O+H+��
��������ˮ���������������������ӣ�ˮ�ⷽ��ʽΪFe3++3H2O?Fe��OH��3+3H+��
�ʴ�Ϊ��Fe3++3H2O?Fe��OH��3+3H+��
���� ������Ҫ�����˻�ѧ����ʽ����д����Ҫע����У����뷽��ʽע��ǿ������ʡ��Ȼ�ѧ����ʽע�����ʵ�״̬����Ӧ�ȵ���ֵ�뵥λ�����ӷ���ʽע��ˮ������ӣ���Ŀ�Ѷ��еȣ�

| �� ���� | IA | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | �� | �� | ||||||
| 3 | �� | �� | �� | �� |
��1���۵�Ԫ�ط�����Mg��
��2���ڡ��ۡ�������Ԫ����Ƚϣ���������ǿ����Na ����дԪ�ط��ţ���
��3��Ԫ�آݺ͢��⻯���У��ȶ��Խ�ǿ����HF�����⻯��ķ���ʽ����
��4���ܵ�����������Ӧ��ˮ����Ļ�ѧʽΪAl��OH��3��
��5������Ԫ�آ۵�ԭ�ӽṹʾ��ͼ
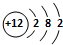 ��
����6��Ԫ�آ۵ĵ��������ᷴӦ�����ӷ���ʽΪMg+2H+=Mg2++H2����
��7��Ԫ�آ��γɵ����磺̼���ƣ���̼�����к���̼����������ʱ����ȥ�����ʵļ����Ǽ��ȣ����ȡ�����ȡ���������
| A�� | �Ȼ�����Һ�ڵ��������µ���������Ӻ������ӵĹ��̽е��� | |
| B�� | ������ˮ���ܵ���������ӵĻ����ﶼ���� | |
| C�� | ������̼����ˮ���ܵ��磬�ʶ�����̼���ڵ���� | |
| D�� | ���ᱵ������ˮ�����ܽⲿ����ȫ���룬�����ᱵ��ǿ����� |
| A�� | �Ͽ�1��N��N����ͬʱ��6��N-H������ | |
| B�� | ���������ܶȲ��� | |
| C�� | ��������ƽ����Է����������� | |
| D�� | N2��H2��NH3������֮��Ϊ1��3��2��״̬ |
| A�� | H2SO4��MgSO4 | B�� | NaCl��Cl2 | C�� | CuSO4��Cu | D�� | HClO��Cl2 |