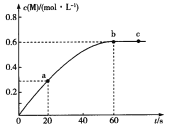
��Ŀ����
����Ŀ��������(Mo)����(W)���ǵڢ�B��Ԫ�أ���ԭ�����������������ǵĵ��ʺͻ�����������������й㷺Ӧ�á��ش��������⣺
��1����Ԫ�ص�����ϼ�Ϊ_________����̬��ԭ�ӵĺ�������Ų������ڻ�̬��ԭ�ӣ����̬��ԭ�Ӻ�����_________��δ�ɶԵ��ӣ���̬��ԭ�ӵĺ�������Ų��ǡ����ع������⣬���̬��ԭ�Ӽ۵��Ӳ�ĵ����Ų�ͼΪ____________________��
��2��������л��ϳɵĴ��������磬����ȩ����ԭ�ɻ������״���
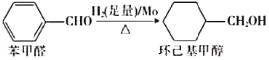
�ٱ���ȩ�����������_________________��ԭ�ӹ�ƽ�档
�ڻ������״������в�ȡsp3�ӻ���ԭ����_____________(дԪ�ط���)��
��3��������(Cr3��)���γɶ�����������[Cr(OH)3(H2O)(H2NCH2CH2NH2)]��
����֪�������������ӵ���λ��ָ��λԭ������������������У�Cr3������λ��Ϊ______��
������������У��ǽ���Ԫ�صĵ縺����С�����˳��Ϊ____________��
��4������һ��������ľ����ṹ��ͼ��ʾ��
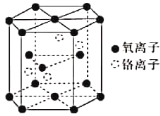
�ٸ�������Ļ�ѧʽΪ____________________��
����֪�������ױ߱߳�Ϊacm����Ϊbcm�������ӵ�������ֵΪNA���þ�����ܶ�Ϊ___________gcm��3(�г�����ʽ)��
���𰸡�+6 6 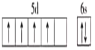 14 C��O 6 H��C��N��O Cr2O3 4
14 C��O 6 H��C��N��O Cr2O3 4![]() ��152/��9NA��a2b��
��152/��9NA��a2b��
��������
��1����Ԫ�صļ۵����Ų�ʽΪ3d54s1��������ϼ�Ϊ+6�ۣ���̬��ԭ�ӵĺ�������Ų������ڻ�̬��ԭ�Ӽ۵����Ų�ʽΪ4d55s1����6��δ�ɶԵ��ӣ���̬��ԭ�ӵĺ�������Ų��ǡ����ع������⣬�۵��Ӳ�ĵ����Ų�ͼΪ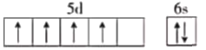 ��
��
��2������������ȩ��ȷ����2��ƽ���غ�ʱ������14ԭ�ӹ�ƽ�棻
�ڻ������״������У�����Cԭ��Ϊ4�����ۼ����µ��Ӷԣ�Oԭ����2������2���µ��Ӷԣ�Ϊsp3�ӻ���
��3����������е�����ΪOH��H2O��H2NCH2CH2NH2������O��Nԭ���ṩ�µ��Ӷ��γ���λ��������λ��Ϊ6��
������������У��ǽ���Ԫ����H��C��N��O���縺�Ե���С�����˳��ΪH��C��N��O��
��4���ٸ��ݸ�������ľ�����֪��Oԭ���ھ����Ķ��㡢�������ĺ����ڣ�����=12��1/6+2��1/2+3=6�������������ڣ�����Ϊ4������֮��Ϊ6��4=3��2����ѧʽΪCr2O3��
����=m/V=��52��2+16��3����2/��a��![]() a��b��NA��=4
a��b��NA��=4![]() ��152/��9NA��a2b����
��152/��9NA��a2b����

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�