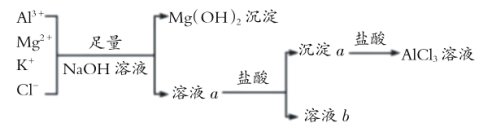
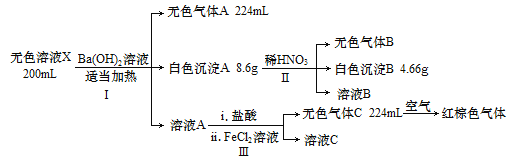
题目内容
【题目】氧化剂和还原剂在生产生活中广泛使用。
(1)H3PO2均可将溶液中的 Ag+还原为 Ag,从而可用于化学镀银。
①H3PO2中P元素的化合价为___。
②利用H3PO2进行化学镀银反应中,氧化剂与还原剂物质的量之比为4:1,则氧化产物为___。
(2)氯酸是一种强酸,氯酸的浓度超过40%就会迅速分解,反应的化学方程式为8HClO3=3O2↑+2Cl2↑+4HClO4+2H2O。根据题意完成下列小题:
①该反应的还原产物是___(填化学式);
②所得混合气体的平均相对分子质量为___。
(3)已知测定锰的一种方法是:锰离子转化为高锰酸根离子,反应体系中有H+、Mn2+、H2O、IO3-、MnO4-、IO4-。
①有关反应的离子方程式为___。
②在锰离子转化为高锰酸根离子的反应中,如果把反应后的溶液稀释到1L,测得溶液的c(H+)=0.03mol/L,则在反应中转移电子的物质的量为___mol。
【答案】+1 H3PO4 Cl2 47.6 2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+ 0.05
【解析】
(1)①化合物中总化合价为0计算出P元素的化合价;②先判断氧化剂、氧化剂,然后根据氧化剂与还原剂的物质的量之比为4:1计算出氧化产物中P的化合价;
(2)得电子的反应物是氧化剂,氧化剂在反应中发生还原反应,失电子的反应物是还原剂,还原剂对应的产物是氧化产物,在数值上,平均式量=平均摩尔质量=![]() ,据此分析;
,据此分析;
(3)锰离子失电子而转化为高锰酸根离子,所以锰离子作还原剂,则得电子化合价降低的物质作氧化剂,根据元素的化合价确定氧化剂和还原产物,再结合离子方程式的书写规则书写;反应后的溶液稀释到1L,测得溶液的pH=2,则n(H+)=1L×0.03mol/L=0.03mol,根据方程式计算。
(1)①H3PO2中,总化合价为0,其中氢元素为+1价,氧元素为-2价,则P元素的化合价为:+1价;故答案为:+1;
②利用H3PO2进行化学镀银反应中,反应中Ag+为氧化剂,H3PO2为还原剂,氧化剂与还原剂的物质的量之比为4:1,设反应产物中P的化合价为x,根据化合价升降相等可得,4×(1-0)=1×(x-1),解得x=5,所以氧化产物为+5价的H3PO4,故答案为:H3PO4;
(2)Cl元素的化合价由+5价→0价,所以还原产物是Cl2;平均式量=平均摩尔质量=![]() =47.6,故答案为:Cl2;47.6;
=47.6,故答案为:Cl2;47.6;
(3)①锰离子失电子被氧化生成高锰酸根离子,所以锰离子作还原剂,氧化剂得电子化合价降低,IO3-和IO4-中碘元素的化合价分别是+5价和+7价,所以IO4-作氧化剂,还原产物是IO3-,同时水参加反应生成氢离子,所以该反应的离子方程式为:
2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+;故答案为:2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+;
②2Mn2++5IO4-+3H2O=2MnO4-+5IO3-+6H+反应中当转移10mol电子时,生成6mol氢离子,如果把反应后的溶液稀释到1L,测得溶液的c(H+)=0.03mol/L,则n(H+)=1L×0.03mol/L=0.03mol,所以转移电子为![]() =0.05mol,故答案为:0.05
=0.05mol,故答案为:0.05