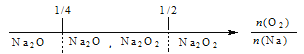��Ŀ����
����Ŀ����һ�������£���3molA��1molB�����������ڹ̶��ݻ�Ϊ2L���ܱ������У��������·�Ӧ��3A(g)+B(g) ![]() xC(g)+2D(g)��2minĩ�÷�Ӧ�ﵽƽ�⣬����0.8mol D�������C��Ũ��Ϊ0.2mol/L�������жϴ������
xC(g)+2D(g)��2minĩ�÷�Ӧ�ﵽƽ�⣬����0.8mol D�������C��Ũ��Ϊ0.2mol/L�������жϴ������
A. x=1
B. B��ת����Ϊ80%
C. 2min��A��ƽ����Ӧ����Ϊ0.3 mol��L-1��min-1
D. �����������ܶȲ��䣬Ҳ����˵���÷�Ӧ�ﵽƽ��״̬
���𰸡�B
��������
A. ƽ��ʱ���ɵ�C�����ʵ���Ϊ0.2molL-1��2L=0.4mol�����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ���0.4mol��0.8mol=x��2�����x=1��A����ȷ��
B. 2minĩ�÷�Ӧ�ﵽƽ�⣬����0.8mol D���ɷ���ʽ3A��g��+B��g��xC��g��+2D��g����֪���μӷ�Ӧ��B�����ʵ���Ϊ��0.8mol��![]() =0.4mol����B��ת����Ϊ
=0.4mol����B��ת����Ϊ![]() ��100%=40%��B�����
��100%=40%��B�����
C. 2min������0.8mol D����2 min��D�ķ�Ӧ����v��D��=![]() =0.2 mol��Lmin��-1������֮�ȵ��ڻ�ѧ������֮�ȣ���v��A��=
=0.2 mol��Lmin��-1������֮�ȵ��ڻ�ѧ������֮�ȣ���v��A��=![]() v��D��=
v��D��=![]() ��0.2 mol��Lmin��-1=0.3 mol��Lmin��-1��C����ȷ��
��0.2 mol��Lmin��-1=0.3 mol��Lmin��-1��C����ȷ��
D. �������ݻ����䣬���������������䣬�ܶ�Ϊ��ֵ��ʼ�ղ��䣬�ʻ��������ܶȲ��䣬����˵����Ӧ�ﵽƽ��״̬��D����ȷ��
��ѡB��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�