��Ŀ����
4����NH4��2SO4�dz��õĻ��ʺͻ���ԭ�ϣ������ֽ⣮ij��ȤС����̽����ֽ������������ϡ���NH4��2SO4��260���400��ʱ�ֽ���ﲻͬ��
��ʵ��̽������С����ѡ����ͼ��ʾװ�ý���ʵ�飨�гֺͼ���װ���ԣ�

ʵ��1������װ��A-B-C-D����������ԣ���ͼʾ�����Լ���װ��Bʢ0.5000mol/L���� 70.00mL��ͨ��N2�ž���������260�����װ��Aһ��ʱ�䣬ֹͣ���ȣ���ȴ��ֹͣͨ��N2��Ʒ����Һ����ɫ��ȡ��װ��B������ָʾ������0.2000moI/LNaOH��Һ�ζ�ʣ�����ᣬ�յ�ʱ����NaOH��Һ25.00mL��������ζ������Һ����SO42-��
��1������X��������Բ����ƿ��
��2���ζ�ǰ�����в�������ȷ˳����dbaec������ĸ��ţ���
a��ʢװ 0.2000mol/LNaOH��Һ b����0.2000mol/L NaOH��Һ��ϴ
c����������¼ d����©����ϴ e���ž��ζ���������ݲ�����Һ��
��3��װ��B����Һ������������ʵ�����0.03mol��
ʵ��2������װ��A-D-B����������ԣ���ͼʾ���¼����Լ���ͨ��N2�ž���������400�����װ��A��
��NH4��2SO4��ȫ�ֽ������ֹͣ���ȣ���ȴ��ֹͣͨ��N2���۲쵽װ��A��D֮��ĵ���������������ɫ���壮�����飬�ð�ɫ�����װ��D����Һ����SO32-����SO42-����һ���о����֣�����������������
��4�����װ��D����Һ����SO32-����SO42-��ʵ�������������ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��B����Һ���յ�������NH3��
��6����NH4��2SO4��400��ֽ�Ļ�ѧ����ʽ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
���� ʵ��1����1������XΪԲ����ƿ��
��2���ζ�ǰ���ȼ��ζ����Ƿ�©ˮ���ٽ�����ϴ��Ȼ���ñ�Һ��ϴ����ע���Һ���ž��ζ���������ݲ�����Һ�棬��������¼���ζ�ǰ����ɣ�
��3�����������������Ƽ���Bװ����ʣ���HCl���μӷ�Ӧ��HCl���շֽ����ɵ�NH3��������Ӧ��NH3+HCl=
NH4Cl��������������NH3�����ʵ�����
ʵ��2����4��ȡD��Һ���Թ��У���������BaCl2��Һ���ټ������ᣬ��ɫ������ȫ�ܽ������ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��D����Һ����SO32-��˵���ֽ�����SO2��װ��A��D֮��ĵ���������������ɫ���壬��ɫ����Ӧ�Ƕ�����������ˮ�γɵ��Σ�װ��B����Һ���յ������ǰ�����
��6���ɣ�5���з�����֪����NH4��2SO4��400��ֽ�ʱ����NH3��SO2��H2O���ɣ�SԪ�ػ��ϼ۽��ͣ����ݵ���ת���غ㣬ֻ��ΪNԪ�ػ��ϼ����ߣ�����������������˵������N2����ƽ��д����ʽ��
��� �⣺��1��������X�Ľṹ��֪��XΪԲ����ƿ���ʴ�Ϊ��Բ����ƿ��
��2���ζ�ǰ���ȼ��ζ����Ƿ�©ˮ���ٽ�����ϴ��Ȼ���ñ�Һ��ϴ����ע���Һ���ž��ζ���������ݲ�����Һ�棬��������¼���ζ�ǰ����ɣ�����ȷ��˳��Ϊ��dbaec��
�ʴ�Ϊ��dbaec��
��3���ζ�ʣ�����ᣬ�յ�ʱ����NaOHΪ0.025L��0.2mol/L=0.005mol����ʣ��HClΪ0.005mol����μӷ�Ӧ��HClΪ0.07L��0.5mol/L-0.005mol=0.03mol���μӷ�Ӧ��HCl���շֽ����ɵ�NH3��������Ӧ��NH3+HCl=NH4Cl��������NH3�����ʵ���Ϊ0.03mol��
�ʴ�Ϊ��0.03��
��4�����װ��D����Һ����SO32-����SO42-��ʵ������������ǣ�ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
�ʴ�Ϊ��ȡ����D��Һ���Թ��У���������BaCl2��Һ���а�ɫ�������ɣ��ټ������ᣬ��ɫ������ȫ�ܽ⣬���ɴ̼�����ζ�����壬˵��D����Һ����SO32-����SO42-��
��5��װ��D����Һ����SO32-��˵���ֽ�����SO2��װ��A��D֮��ĵ���������������ɫ���壬��ɫ����Ӧ�Ƕ�����������ˮ�γɵ��Σ�װ��B����Һ���յ������ǰ�����
�ʴ�Ϊ��NH3��
��6���ɣ�5���з�����֪����NH4��2SO4��400��ֽ�ʱ����NH3��SO2��H2O���ɣ�SԪ�ػ��ϼ۽��ͣ����ݵ���ת���غ㣬ֻ��ΪNԪ�ػ��ϼ����ߣ�����������������˵������N2���ֽⷴӦ����ʽΪ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
�ʴ�Ϊ��3��NH4��2SO4$\frac{\underline{\;400��\;}}{\;}$4NH3��+3SO2��+6H2O��+N2����
���� ���⿼�黯ѧʵ�飬�漰��ѧ�������ζ�������ʵ�鷽����ơ���ѧ���㡢�����ƶϡ���ѧ����ʽ��д�ȣ��Ƕ�ѧ���ۺ������Ŀ��飬�ϺõĿ���ѧ����������������֪ʶǨ�����������Ѷ��еȣ�

 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�| A�� | ��֬�Ʒ���ʵ���м�һ�������Ҵ���Ϊ��������֬���ܽ⣬�ӿ췴Ӧ���� | |
| B�� | ��֬�Ʒ���ʵ���м�һ�����ı���ʳ��ˮΪ�˽��ͷ������ܽ�ȣ�ʹ�������� | |
| C�� | ��Ӧ�����У����Һ�Ƿ�ֲ�����ж�������Ӧ�Ƿ���ȫ | |
| D�� | ������Ӧ�IJ����ж��и��� |
| A�� | �轺������ʳƷ����� | |
| B�� | P2O5��������ʳƷ����� | |
| C�� | ��ˮ���Ȼ��ƿ�����ʳƷ����� | |
| D�� | �ӹ��������ˮ�Ե�ֲ����ά������ʳƷ����� |
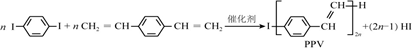
����˵����ȷ���ǣ�������
| A�� | �ϳ�PPV�ķ�ӦΪ�Ӿ۷�Ӧ | |
| B�� | PPV��۱���ϩ������ͬ���ظ��ṹ��Ԫ | |
| C�� |  �ͱ���ϩ��Ϊͬϵ�� �ͱ���ϩ��Ϊͬϵ�� | |
| D�� | ͨ�������ⶨPPV��ƽ����Է����������ɵ���ۺ϶� |
| A�� | ��������ˮ�� | B�� | ������������ʪ�� | ||
| C�� | Ư�۾��������� | D�� | ������ʳƷ���ڵ������� |
| A�� |  �۲�Fe��OH��2������ �۲�Fe��OH��2������ | |
| B�� |  ����һ�����ʵ���Ũ�ȵ�NaNO3��Һ ����һ�����ʵ���Ũ�ȵ�NaNO3��Һ | |
| C�� |  ʵ������ȡ�� ʵ������ȡ�� | |
| D�� |  ��֤��ϩ������ ��֤��ϩ������ |
| A�� | ��Ƥ��--��ҵ | B�� | ���Ͻ�Ƭ--ұ��ҵ | ||
| C�� | Ǧ��о--��ƹ�ҵ | D�� | Ǧ����--Ϳ�Ϲ�ҵ |
| A�� | �������Ż�ʱʹ����ĭ�������� | |
| B�� | ���Թܼ���̼�����ƹ���ʱʹ�Թܿ���ֱ���� | |
| C�� | Ũ���ὦ��Ƥ����ʱ������ϡ����������Һ��ϴ | |
| D�� | �Ʊ���ϩʱ���Ҵ���Ũ����Ļ��Һ�м������Ƭ |
| A�� | H��D��T������ĺ��������ڱ��е�λ�ò�ͬ | |
| B�� | Ԫ�ؼ���̬�⻯��Խ�ȶ�����ǽ�����Խǿ | |
| C�� | ������Ӳ�ṹ��ͬ�����ӣ��˵����Խ�������Ӱ뾶ҲԽ�� | |
| D�� | ��A���AԪ���γɵĻ����ﶼ�����ӻ����� |