��Ŀ����
12����֪�����£�0.1mol•L?1CH3COONH4��Һ�����ԣ������й�������ȷ���ǣ�������| A�� | ����Һ���ɵ������Ũ�Ⱦ�Ϊ0.1mol•L?1�Ĵ���Ͱ�ˮ��Ӧ�õ� | |
| B�� | CH3COONH4���봿ˮ�У�ˮ�ĵ���̶Ȳ��� | |
| C�� | �����£����볣��K��CH3COOH��=K��NH3•H2O�� | |
| D�� | ����Һ��c��CH3COO?������ͬŨ��CH3COONa��Һ�е�c��CH3COO?�� |
���� 0.1mol•L-1 CH3COONH4��Һ�����ԣ�˵�������������笠����ӵ�ˮ��̶���ͬ����ͬ���ʵ����Ĵ�����һˮ�ϰ��к����ɴ���泥������������笠�����ˮ����ٽ���ˮ�ĵ���̶�����K��CH3COOH��=$\frac{�������ƽ�ⳣ��}{Kw}$��K��NH3•H2O��=$\frac{笠�ˮ��ƽ�ⳣ��}{Kw}$���ݴ˷�����
��� �⣺A���������Ũ�Ⱦ�Ϊ0.1mol•L-1�Ĵ���Ͱ�ˮ��Ӧ�õ�0.05mol•L-1 CH3COONH4��Һ����A����
B�������������笠�����ˮ����ٽ���ˮ�ĵ���̶�����B����
C��K��CH3COOH��=$\frac{Kw}{�������ˮ��ƽ�ⳣ��}$��K��NH3•H2O��=$\frac{Kw}{笠���ˮ��ƽ�ⳣ��}$��0.1mol•L-1 CH3COONH4��Һ�����ԣ�˵�������������笠����ӵ�ˮ��̶���ͬ������������ӵ�ˮ��ƽ�ⳣ��=笠����ӵ�ˮ��ƽ�ⳣ����ˮ�����ӻ�������ͬ������K��CH3COOH��=K��NH3•H2O������C��ȷ��
D��CH3COONa��Һ�е�c��CH3COO-����Ȼˮ�⣬�������Һ�д����������笠�����ˮ����ٽ�������Һ��c��CH3COO-��С��ͬŨ��CH3COONa��Һ�е�c��CH3COO-������D����
��ѡC��
���� ���⿼��������ˮ���Ӱ�����ء�����ƽ�ⳣ����ˮ��ƽ�ⳣ���Լ�ˮ�����ӻ��Ĺ�ϵ��ע�����������������������ˮ����ٽ�����Ŀ�Ѷ��еȣ�

 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�| A�� | ���������ᵼ������⻯ѧ������Σ���ϴ� | |
| B�� | ����Ʒ��������ʢ���ᡢ����Һ�����˳�ʱ��ʢ���̲˵�����ʳƷ | |
| C�� | �����м�������NO ��ٽ�Ѫ�����ţ���ֹѪ��˨�� | |
| D�� | �������ȷ�Ӧ��ʵ�ֹ�ҵ��þ |
| A�� | ��Ӧֹͣ�� | B�� | ����Ӧ�������淴Ӧ���ʾ�Ϊ�� | ||
| C�� | ��Ӧ���������Ũ����� | D�� | ����Ӧ�������淴Ӧ������� |
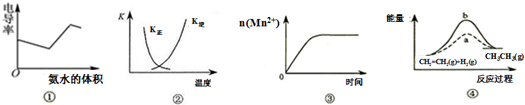
| A�� | ͼ�ٱ�ʾ25��ʱ��������ʹ�������Һ�е��백ˮ�����е絼�ʣ�������Һ����������С�����������ı仯��ϵ | |
| B�� | ͼ�������߱�ʾ��Ӧ2SO2��g��+O2��g���T2SO3��g������H��0 �����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯 | |
| C�� | ͼ�۱�ʾ10mL 0.01mol•L-1 KMnO4 ������Һ�������0.1mol•L-1 H2C2O4��Һ���ʱ��n��Mn2+�� ��ʱ��ı仯 | |
| D�� | ͼ����a��b���߷ֱ��ʾ��ӦCH2=CH2��g��+H2��g����CH3CH3��g������H��0ʹ�ú�δʹ�ô���ʱ����Ӧ�����е������仯 |
| A�� | FeCl3 | B�� | CuSO4 | C�� | FeSO4 | D�� | NaCl |
 ���з�Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g����H��0����ͼ��ʾ��Ӧ����t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ij�������������仯���������ͼ��t2ʱ�̷����ı������������B��
���з�Ӧ��CO2��g��+H2��g��?CO��g��+H2O��g����H��0����ͼ��ʾ��Ӧ����t1ʱ�̴ﵽƽ�⣬��t2ʱ����ı�ij�������������仯���������ͼ��t2ʱ�̷����ı������������B��
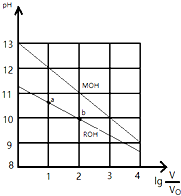 25��ʱ��Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��MOH��ROH��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{V}{{V}_{0}}$�ı仯��ͼ��ʾ��
25��ʱ��Ũ�Ⱦ�Ϊ0.10mol/L�������ΪV0��MOH��ROH��Һ���ֱ��ˮϡ�������V��pH��lg$\frac{V}{{V}_{0}}$�ı仯��ͼ��ʾ��