��Ŀ����
2����֪�±����ݣ�| ���� | �۵�/�� | �е�/�� | �ܶ�/��g/cm3�� |
| �Ҵ� | -144 | 78 | 0.789 |
| ���� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.90 |
| Ũ���� | -- | 338 | 1.84 |
��1��д���÷�Ӧ�Ļ�ѧ��ӦʽCH3COOH+HOCH2CH3$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2����װ��ͼ��װ���������ڴ��Թ������ƺ������Ϊ3��2���Ҵ������ᣬ���������ڣ��ܾ�Ҳû�й���ζҺ�����ɣ�ԭ����©��Ũ���ᣮ
��3�������ϱ����ݷ�����Ϊʲô�Ҵ���Ҫ����һЩ����ԭ�����Ҵ��ķе�ͣ��ӷ�����ģ�
����ȷ��������ʵ�飬��ѧ���ܿ���С�Թ����ռ������������������ᡢ�Ҵ��Ļ���������뺬���ᡢ�Ҵ���ˮ�����������ֲ�Ʒ����ͼ�Ƿ��������������ͼ����ͼ��Բ���ű�ʾ�����ʵ����Լ�����ű�ʾ�ʵ��ķ��뷽����
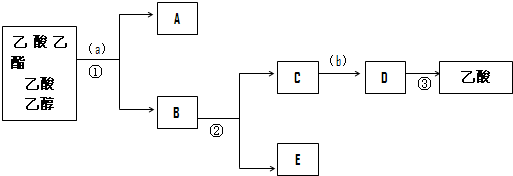
д��������Լ����Լ���a���DZ���̼���ƣ��Լ���b����ϡ������Һ
д���йصIJ������뷽�������Ƿ�Һ����������������
��6���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ���dz�ȥ���������л��е�����ˮ��
���� ���뱥��̼����a�ܽ��Ҵ������ᷴӦ���ɴ�������Һ������������̼������Һ�в��ֲܷ��Һ���������������������������Һ������Ҵ���Ȼ����ʣ����Һ�м���ϡ����b��ϡ����������Ʒ�Ӧ���ɴ���������ƻ����Һ����������������
��1��������Ҵ���Ũ������·�Ӧ��������������
��2��������Ӧ��ҪŨ������������
��3�����ݱ������ݿ�֪�Ҵ��е���ӷ���
��4���������ʵ�ԭ�������Լ������������ҩƷ��Ӧ�������������ʣ�������ҩƷ�����뱻�����ҩƷ�ֿ������뱥��̼����a�ܽ��Ҵ������ᷴӦ���ɴ�������Һ������������̼������Һ�в��ֲܷ��Һ���������������������������Һ������Ҵ���Ȼ����ʣ����Һ�м���ϡ����b��ϡ����������Ʒ�Ӧ���ɴ���������ƻ����Һ��������������������ͼʾ���̿��Dz���������
��5����ˮ̼���Ʒ�ĩ��һ�����õ���ˮ�������Գ������������л��е�����ˮ��
��� �⣺��1���������Ҵ���Ũ���������¼��ȷ���������Ӧ��������������ˮ��ͬʱ�÷�Ӧ���棬��Ӧ�Ļ�ѧ����ʽΪCH3COOH+CH3CH2OH$\stackrel{Ũ����}{?}$CH3COOC2H5+H2O��
�ʴ�Ϊ��CH3COOH+HOCH2CH3$?_{��}^{Ũ����}$CH3COOCH2CH3+H2O��
��2�������Ҵ���Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ�����������������������Ӧ���ʴ�Ϊ��©��Ũ���
��3�����ݱ������ݿ�֪�Ҵ��е���ӷ�������Ӧ����һЩ���ʴ�Ϊ���Ҵ��ķе�ͣ��ӷ�����ģ�
��4�����뱥��̼������Һa���ܽ��Ҵ������ᷴӦ���ɴ�������Һ������������̼������Һ�в��ֲܷ��Һ���������������������������Һ������Ҵ���Ȼ����ʣ����Һ�м���ϡ����b��ϡ����������Ʒ�Ӧ���ɴ���������ƻ����Һ����������������
�ʴ�Ϊ������̼���ƣ�ϡ������Һ����Һ����������
��5���ڵõ���A�м�����ˮ̼���Ʒ�ĩ����Ŀ���ǣ���ȥ���������л��е�����ˮ���ʴ�Ϊ����ȥ���������л��е�����ˮ��
���� ���⿼����ʵ�����Ʊ�������������Ҫ�����л����ƶϡ��л���ķ����ᴿ�������������Ʊ��ȣ����ؿ����л�������ᴿ���ؼ�����������ᴿԭ�����Ѷ��еȣ�

| ѡ�� | ʵ����� | ���� | ���� |
| A | ����Fe��OH��2¶���ڿ�����һ��ʱ�� | ��ɫ����Ѹ�ٱ�Ϊ����ɫ�����ձ�Ϊ���ɫ | ˵��Fe��OH��2�ױ�������Fe��OH��3 |
| B | �����½�FeƬ����Ũ������ | �����Ա仯 | Fe��Ũ�����Ӧ |
| C | ��һС��Na����ҽ�þƾ��� | �������� | Naֻ�û������ǻ��ϵ��� |
| D | ��ij��ɫ��Һ�еμ���ˮ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ����I- |
| A�� | A | B�� | B | C�� | C | D�� | D |
| 1 | 2 | 3 | 4 | 5 | 6 | �� |
| CH4 | C2H6 | C5H12 | C8H18 | C17H36 | �� | �� |
| A�� | C16H34 | B�� | C22H46 | C�� | C26H54 | D�� | C27H56 |
| X | Y | ||
| Z | W |
| A�� | XԪ��Z���γ���̬�⻯��XH3 | B�� | Z��W�γɵĻ�����������ʱ�ܵ��� | ||
| C�� | ��Ȼ����ڴ����ĵ���Z | D�� | X��Y�γɵĻ����ﲻ����3�� |
| A�� | 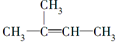 +Br2��CCl4���� +Br2��CCl4���� | |
| B�� | CH2=CH-CH2-CH3+HCl$��_{��}^{����}$ | |
| C�� | CH3-CH=CH2+H2O$��_{���ȡ���ѹ}^{����}$ | |
| D�� | CH4+Cl2$\stackrel{����}{��}$ |
| A�� | ��������ƽ����ҩƷʱ�����̷�ҩƷ�����̷����� | |
| B�� | ���Թ��еμ��Լ�ʱ�����ι��¶˽����Թ��ڱ� | |
| C�� | ��ȼ�ŵľƾ���ȥ��ȼ��һ�ƾ��� | |
| D�� | ʹ����ֽ������Һ������ʱ������ֽ������Һ�� |
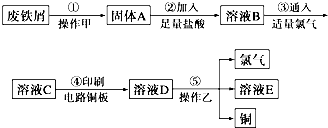
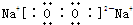 ��
��