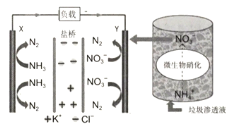
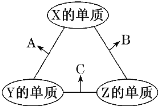
��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
�� ���� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
(1)ԭ�Ӱ뾶��С��Ԫ����___(��Ԫ������)��д�����ԭ�ӽṹʾ��ͼ____________��
(2)����������Ӧ��ˮ�����У�������ǿ����___(�û�ѧʽ�ش���ͬ)��������ǿ����____��
(3)������γɵĻ������У���ѧ������Ϊ____��
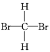
(4)������γɵĻ�����ĵ���ʽΪ__��������γɵ�ԭ�Ӹ�����Ϊ1:2�Ļ�����ĽṹʽΪ__��
(5)������γɵ�һ����������ƽ���������νṹ����Է�������Ϊ78��д����������ȡ����Ӧ�Ļ�ѧ����ʽ(��дһ������)��_��
(6)Ϊ̽��Ԫ�آں͢�ķǽ�����ǿ����ijͬѧ�������ͼ��ʾ��װ�ý���ʵ��(�г���������ȥ��װ������������)����ش�
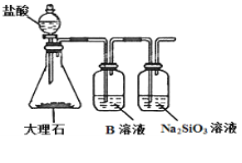
����ҺBΪ______��B��Һ��������______��
��������_______������֤������_______(�û�ѧʽ�ش�)����ǽ�����______(��Ԫ�ط��Żش�)��
���𰸡��� 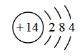 NaOH HClO4 ���ۼ�
NaOH HClO4 ���ۼ� ![]() O=C=O
O=C=O ![]() +Br2
+Br2![]()
![]() +HBr����
+HBr����![]() +HO-NO2(Ũ)
+HO-NO2(Ũ)![]()
![]() +H2O ����NaHCO3��Һ ��ȥCO2�����е�HCl Na2SiO3��Һ�в�����ɫ��״��������Na2SiO3��Һ����ǣ� H2CO3 > H2SiO3 C > Si
+H2O ����NaHCO3��Һ ��ȥCO2�����е�HCl Na2SiO3��Һ�в�����ɫ��״��������Na2SiO3��Һ����ǣ� H2CO3 > H2SiO3 C > Si
��������
��Ԫ�������ڱ���λ�ã���֪��ΪH����ΪC����ΪN����ΪO����ΪF����ΪNa����ΪMg����ΪSi����ΪCl��
(1)����Ԫ����Hԭ�Ӱ뾶��С��Ԫ������Ϊ�⣬�ദ�ڵ������ڵ�IVA�壬��Ϻ�������Ų����ɣ��˵����Ϊ14����ԭ�ӽṹʾ��ͼΪ��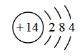 ���ʴ�Ϊ���⣻
���ʴ�Ϊ���⣻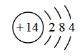 ��
��
(2)ͬ����������ҽ����Լ�����ͬ�������϶��½�������ǿ���ʱ���Ԫ��Na�Ľ�������ǿ����NaOH�ļ�����ǿ��FԪ��û����ۺ����ᣬ������ǿ����ۺ�����ΪHClO4���ʴ�Ϊ��NaOH��HClO4��
(3)������γɵĻ�����ΪHF�������ǽ���ԭ��֮���γɹ��ۼ����ʴ�Ϊ�����ۼ���
(4)������γɵĻ�����ΪMgCl2����þ�����������ӹ��ɣ������ʽΪ![]() ��������γɵ�ԭ�Ӹ�����Ϊ1:2�Ļ�����ΪCO2��������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪ��O=C=O���ʴ�Ϊ��
��������γɵ�ԭ�Ӹ�����Ϊ1:2�Ļ�����ΪCO2��������Cԭ����Oԭ��֮���γ�2�Թ��õ��Ӷԣ���ṹʽΪ��O=C=O���ʴ�Ϊ��![]() ��O=C=O��
��O=C=O��
(5)����ƽ���������νṹ����Է�������Ϊ78�����������DZ���������Һ�巢��ȡ����Ҳ����Ũ����ȷ���ȡ������ѧ����ʽΪ��![]() +Br2
+Br2![]()
![]() +HBr����
+HBr����![]() +HO-NO2(Ũ)
+HO-NO2(Ũ)![]()
![]() +H2O���ʴ�Ϊ
+H2O���ʴ�Ϊ![]() +Br2
+Br2![]()
![]() +HBr����
+HBr����![]() +HO-NO2(Ũ)
+HO-NO2(Ũ)![]()
![]() +H2O��
+H2O��
(6)Ԫ�صķǽ�����Խǿ������ۺ����������Խǿ��ʵ������ǿ���Ʊ�����ԭ���������ӷ������ɵĶ�����̼����HCl�����ʵ�飬B���Լ�������Ϊ��ȥCO2�����е�HCl���ñ��͵� NaHCO3��Һ��װ���������Լ�ƿ�з���������̼������Ƶķ�Ӧ���ɹ��ᣬ���в�����ɫ��״������˵������H2CO3>H2SiO3���ǽ�����C>S����Ӧ��ѧ����ʽΪCO2+H2O+Na2SiO3=Na2CO3+H2SiO3��
�ʴ�Ϊ���ٱ��͵� NaHCO3��Һ����ȥCO2�����е�HCl����Na2SiO3��Һ�в�����ɫ��״��������Na2SiO3��Һ����ǣ���H2CO3 > H2SiO3��C > Si��

����Ŀ��һ���¶��£������������Ϊ0.5 L�ĺ����ܱ������з�����Ӧ��CO(g)+Cl2(g)![]() COCl2(g)�������������з�Ӧ��5 minʱ�ﵽƽ��״̬��
COCl2(g)�������������з�Ӧ��5 minʱ�ﵽƽ��״̬��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | ||
CO | Cl2 | COCl2 | COCl2 | ||
�� | 500 | 1.0 | 1.0 | 0 | 0.8 |
�� | 500 | 1.0 | a | 0 | 0.5 |
�� | 600 | 0.5 | 0.5 | 0.5 | 0.7 |
����˵������ȷ����
A. ��������ǰ5 min��ƽ����Ӧ����v(CO)=0.16 mol��L-1��min-1
B. �÷�Ӧ����ӦΪ���ȷ�Ӧ
C. ����������ʼʱCl2�����ʵ���Ϊ0.55 mol
D. ����ʼʱ�����������CO0.8mol��Cl20.8mol���ﵽƽ��ʱCOת���ʴ���80%