��Ŀ����
����Ŀ����ȩ(CH3CHO)���л��ϳ��еĶ�̼�Լ����Ǻϳ����ᡢ�Ҵ�������������ũҩDDT�ȵ�ԭ�ϡ��ش���������:
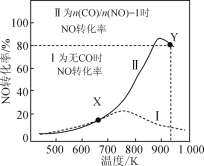
(1)Andrea Dasic ������ڽ�������M��������N2OΪ����������������ϩ������ȩ������ϵ������ԭѭ����ͼ��ʾ��(��������ԭ�ӵĽ������OA��ʾ)

��ԭ����N����NO�Ľ����OA(N)= 167.4kJ��mol-1,��ԭ������ϩ������ȩ�Ľ����OA(C2 H4)=473 kJmol-1,��������÷�Ӧ�����Ľ���M����ԭ�ӵĽ����OA(M)��ֵӦ����:_______,ʹ�ô�����ʹ�÷�Ӧ�Ļ��____(����������������С��)��
(2)��֪CO(g)��CH4(g)��CH3CHO(l)��ȼ���ȷֱ�Ϊ283.0 kJmol-1��890.31 kJ�� mol-1��1167.9 kJmol-1,����ȩ�ķֽⷴӦCH3CHO(l) ![]() CH4(g)+CO(g)�� H =________��
CH4(g)+CO(g)�� H =________��
(3)��֪:�ں�������I2����Һ��,��ӦCH3CHO(aq) ![]() CH4 (g)+CO(g)����������:
CH4 (g)+CO(g)����������:
��I����ӦΪCH3CHO(aq) +I2(aq)��CH3I(l) + HI(aq) +CO(g)(����Ӧ),��II��Ϊ�췴Ӧ��
����д����II����Ӧ�Ļ�ѧ����ʽ:__________��
������I2��Ũ��______(������"��������")���������ܷ�Ӧ��ƽ������,����Ϊ_________��
(4)��ȩ�����뱥�͵�NaHSO3��Һ������Ӧ����ˮ���Ե�![]() -�ǻ�������:CH3CHO+ NaHSO3
-�ǻ�������:CH3CHO+ NaHSO3![]() CH3CH(OH)SO3Na(
CH3CH(OH)SO3Na(![]() -�ǻ�����Ϊ������ˮ��ǿ��)����Ӧ�ﵽƽ���,��������������,��Ӧ��ϵ�м����������ᣬƽ�⽫___ (���� ��������������������")�ƶ���
-�ǻ�����Ϊ������ˮ��ǿ��)����Ӧ�ﵽƽ���,��������������,��Ӧ��ϵ�м����������ᣬƽ�⽫___ (���� ��������������������")�ƶ���
(5)��100~120 ��C��PdCl2 �C CuCl2����������,��ϩ������O2��Ӧ������ȩ: 2CH2=CH2(g) +O2(g) ![]() 2CH3CHO(g)�� T��Cʱ����2 L�ĺ����ܱ�������ͨ��3 mol CH2=CH2(g)��3 mol O2(g),����������Ӧ,��Ӧ�պôﵽƽ��״̬����ϵѹǿ��Ϊ��ʼѹǿ��5/6����CH2=CH2(g)��ƽ��ת����Ϊ____ (�������3λ��Ч����),T ��Cʱ�÷�Ӧ��ƽ�ⳣ��KΪ________��
2CH3CHO(g)�� T��Cʱ����2 L�ĺ����ܱ�������ͨ��3 mol CH2=CH2(g)��3 mol O2(g),����������Ӧ,��Ӧ�պôﵽƽ��״̬����ϵѹǿ��Ϊ��ʼѹǿ��5/6����CH2=CH2(g)��ƽ��ת����Ϊ____ (�������3λ��Ч����),T ��Cʱ�÷�Ӧ��ƽ�ⳣ��KΪ________��
���𰸡�167.4kJ��mol-1��OA(M) ��473 kJmol-1 ��С +5.41 kJ��mol1 CH3I(l) + HI(aq) �� CH4(g) + I2(aq) �� �ܷ�Ӧ��ƽ������������Ӧ������I2������Ӧ�ķ�Ӧ������I2��Ũ�ȣ�����Ӧ���������ܷ�Ӧ��ƽ���������� ���� 66.7% 4
��������
���ɴ��������û������ͼʾ��Ϣ֪������ԭ��������Ľ���������м�ֵʱ���˷�Ӧ�ɷ����������ή�ͷ�Ӧ�Ļ�ܡ�
�Ʒֱ�д��ȼ���ȵ��ȷ�Ӧ����ʽ�����õ�3�����ȷ�Ӧ����ʽ��ȥ��1���͵�2�����ȷ�Ӧ����ʽ��
�������ݵ�I����Ӧ�ʹ����ķ�Ӧԭ�����ܷ�Ӧ��ȥ��I����Ӧ�õ���II����Ӧ�Ļ�ѧ����ʽ�����ܷ�Ӧ����������Ӧ���ʾ�����I2������Ӧ�ķ�Ӧ��������I2��Ũ�������������ܷ�Ӧ��ƽ�����ʡ�
����Ӧ��ϵ�м����������ᣬ���������NaHSO3��Ӧ��ʹ��Ӧ��NaHSO3��Ũ�ȼ�С��ƽ�������ƶ���
�ɽ�������ʽ������ѹǿ֮�ȵ������ʵ���֮�Ƚ��м��㣬����ת���ʺ�ƽ�ⳣ����
����ԭ����N����NO�Ľ����OA(N) = 167.4 kJ��mol1����ԭ������ϩ������ȩ�Ľ����OA(C2H4)=473 kJ��mol1���ɴ��������û������ͼʾ��Ϣ֪������ԭ��������Ľ���������м�ֵʱ���˷�Ӧ�ɷ�������˿������÷�Ӧ�����Ľ���M����ԭ�ӵĽ����OA(M)��ֵӦ����167.4kJ��mol-1��OA(M) ��473 kJmol-1�������ή�ͷ�Ӧ�Ļ�ܣ��ʴ�Ϊ��167.4 kJ��mol1��OA(M) ��473 kJ��mol1����С��
����֪CO(g)��CH4(g)��CH3CHO(l)��ȼ���ȷֱ�Ϊ283.0 kJ��mol1��890.31 kJ��mol1��1 167.9 kJ��mol1���ֱ�д��ȼ���ȵ��ȷ�Ӧ����ʽ�����õ�3�����ȷ�Ӧ����ʽ��ȥ��1���͵�2�����ȷ�Ӧ����ʽ������ȩ�ķֽⷴӦCH3CHO(l) ![]() CH4(g)+CO(g)�� H =1167.9 kJ��mol1 (283.0 kJ��mol1) (890.31 kJ��mol1) = +5.41 kJ��mol1���ʴ�Ϊ��+5.41 kJ��mol1��
CH4(g)+CO(g)�� H =1167.9 kJ��mol1 (283.0 kJ��mol1) (890.31 kJ��mol1) = +5.41 kJ��mol1���ʴ�Ϊ��+5.41 kJ��mol1��
�������ݵ�I����Ӧ�ʹ����ķ�Ӧԭ�����ܷ�Ӧ��ȥ��I����Ӧ�õ���II����Ӧ�Ļ�ѧ����ʽCH3I(l) + HI(aq) �� CH4(g) + I2(aq)���ʴ�Ϊ��CH3I(l) + HI(aq) �� CH4(g) + I2(aq)��
���ܷ�Ӧ����������Ӧ���ʾ�����I2������Ӧ�ķ�Ӧ��������I2��Ũ�������������ܷ�Ӧ��ƽ�����ʣ��ʴ�Ϊ���ܣ��ܷ�Ӧ��ƽ������������Ӧ������I2������Ӧ�ķ�Ӧ������I2��Ũ�ȣ�����Ӧ���������ܷ�Ӧ��ƽ����������
�ȷ�Ӧ�ﵽƽ����������������䣬��Ӧ��ϵ�м����������ᣬ���������NaHSO3��Ӧ��ʹ��Ӧ��NaHSO3��Ũ�ȼ�С��ƽ�������ƶ����ʴ�Ϊ������
��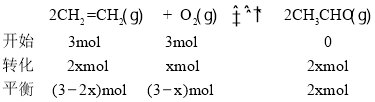
![]() �����x = 1mol����CH2=CH2��ƽ��ת����Ϊ
�����x = 1mol����CH2=CH2��ƽ��ת����Ϊ![]() ��T ��Cʱ�÷�Ӧ��ƽ�ⳣ��
��T ��Cʱ�÷�Ӧ��ƽ�ⳣ��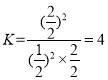 ���ʴ�Ϊ��66.7%��4��
���ʴ�Ϊ��66.7%��4��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ٣����ڱ��е�λ�ã��û�ѧ����ش��������⣺
�� ���� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
(1)ԭ�Ӱ뾶��С��Ԫ����___(��Ԫ������)��д�����ԭ�ӽṹʾ��ͼ____________��
(2)����������Ӧ��ˮ�����У�������ǿ����___(�û�ѧʽ�ش���ͬ)��������ǿ����____��
(3)������γɵĻ������У���ѧ������Ϊ____��
(4)������γɵĻ�����ĵ���ʽΪ__��������γɵ�ԭ�Ӹ�����Ϊ1:2�Ļ�����ĽṹʽΪ__��
(5)������γɵ�һ����������ƽ���������νṹ����Է�������Ϊ78��д����������ȡ����Ӧ�Ļ�ѧ����ʽ(��дһ������)��_��
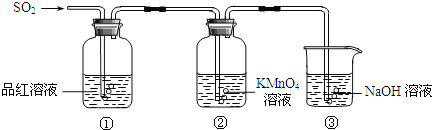
(6)Ϊ̽��Ԫ�آں͢�ķǽ�����ǿ����ijͬѧ�������ͼ��ʾ��װ�ý���ʵ��(�г���������ȥ��װ������������)����ش�
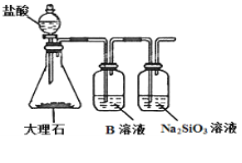
����ҺBΪ______��B��Һ��������______��
��������_______������֤������_______(�û�ѧʽ�ش�)����ǽ�����______(��Ԫ�ط��Żش�)��