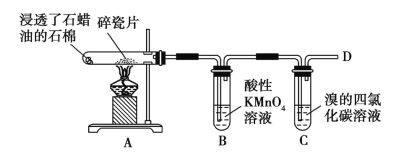
��Ŀ����
����Ŀ��CH3CH=CHCH3��������ת����ϵ��

��1���������ͬ���칹��Ľṹ��ʽΪ________��
��2��д����ͼ�з�Ӧ���ڴ����ͼ��������µĻ�ѧ����ʽ��________��
��3��CH3CH=CHCH3��ʹ��ˮ������KMnO4��Һ��ɫ��������ɫ��ԭ����ͬ��˵��ԭ��_______��
��4��ϩ��A��CH3CH=CHCH3��һ��ͬ���칹�壬���ڴ�����������������Ӧ�IJ��ﲻ�������飬��A�Ľṹ��ʽΪ___________��A�����й�ƽ���̼ԭ�Ӹ���Ϊ___________��
��5����Ӧ�ڵIJ����ͬ���칹����______�֡�
���𰸡�CH(CH3)3 CH3CH=CHCH3��H2![]() CH3CH2CH2CH3 ��ͬ��ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ��ʹ����KMnO4��Һ��ɫ�Ƿ�����������Ӧ CH2=C(CH3)2 4 3
CH3CH2CH2CH3 ��ͬ��ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ��ʹ����KMnO4��Һ��ɫ�Ƿ�����������Ӧ CH2=C(CH3)2 4 3
��������
��1��������CH3CH2CH2CH3����̼���칹��ͬ���칹�����춡��CH(CH3)3��
��2��CH3CH=CHCH3�������ڴ��������·����ӳɷ�Ӧ����CH3CH2CH2CH3����Ӧ����ʽ��CH3CH=CHCH3��H2![]() CH3CH2CH2CH3��
CH3CH2CH2CH3��
��3��CH3CH=CHCH3ʹ��ˮ��ɫ�Ƿ����˼ӳɷ�Ӧ��ʹ����KMnO4��Һ��ɫ�Ƿ�����������Ӧ��������ɫ��ԭ����ͬ��
��4��CH3CH=CHCH3��ͬ���칹����CH2=CHCH2CH3��CH2=C(CH3)2��ǰ���������ӳɵIJ����������飬���AΪCH2=C(CH3)2��A�����к���̼̼˫������̼̼˫���е�̼ԭ��ֱ��������ԭ�Ӽ�̼̼˫���е�����̼ԭ�ӣ���6��ԭ�ӿ϶���ƽ�棬��A�����й�ƽ���̼ԭ�Ӹ���Ϊ4��
��5����Ӧ���IJ����ͬ���칹����CH3CH2CH2CH2Br��(CH3)2CHCH2Br��(CH3)3CBr����3�֡�

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ij��ҵ�����У����뷴Ӧ���Ļ��������NO��O2�����ʵ��������ֱ�Ϊ0.10��0.06��������ѧ��Ӧ2NO(g)+O2(g)=2NO2(g)��������������ͬʱ�����ʵ���������±���
ѹǿ/(��105Pa) | �¶�/�� | NO�ﵽ����ת������Ҫʱ��/s | ||
50% | 90% | 98% | ||
1.0 | 30 | 12 | 250 | 2830 |
90 | 25 | 510 | 5760 | |
8.0 | 30 | 0.2 | 3.9 | 36 |
90 | 0.6 | 7.9 | 74 | |
���ݱ������ݣ�����˵����ȷ����
A. �����¶ȣ���Ӧ���ʼӿ�
B. ����ѹǿ����Ӧ���ʱ���
C. ��1.0��105Pa��90�������£���ת����Ϊ98%ʱ�ķ�Ӧ�Ѵﵽƽ��
D. �����뷴Ӧ���Ļ������Ϊamol����Ӧ������v=��n/��t��ʾ������8.0��105Pa��30��������ת���ʴ�50%����90%ʱ��NO�ķ�Ӧ����Ϊ4a/370mol/s