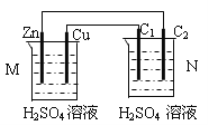
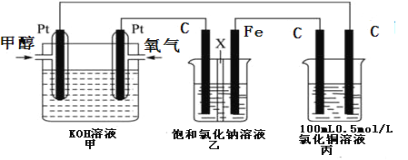
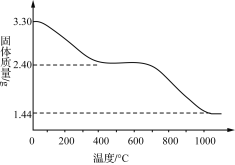
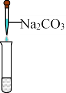
��Ŀ����
����Ŀ���л���A��F֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ��������B����Է���������D��2����
��ʾ��![]() ���Զ���ˮ�γ�R��CHO��
���Զ���ˮ�γ�R��CHO��
����������Ϣ�ش��������⣺
��1��B�ķ���ʽ��________________��
��2������D�й����ſ�ʹ�õ��Լ�������_________��д��D������ͬϵ������Լ���Ӧ�Ļ�ѧ����ʽ_______________________________��
��3��C��FeCl3��Һ����ɫ���˴Ź������������ĸ��壬��������Ϊ1:2:2:1��д��C�Ľṹ��ʽ_______________________��
��4��д����������������C��ͬ���칹��Ľṹ��ʽ_______��______��______��________��
�ٱ����ϴ��ڶ�λȡ����������FeCl3��Һ����ɫ��������������Һ��Ӧ��
��5��д��A�Ľṹ��ʽ__________________________��
��6����1molA������NaOH��Ӧ�����������___________mol NaOH��
���𰸡�C2H4O2 ������Һ������Cu��OH��2����Һ CH3CHO+2Ag��NH3��2OH![]() CH3COONH4+2Ag+3NH3��+H2O��CH3CHO+NaOH+2Cu��OH��2
CH3COONH4+2Ag+3NH3��+H2O��CH3CHO+NaOH+2Cu��OH��2![]() CH3COONa+Cu2O��+3H2O
CH3COONa+Cu2O��+3H2O ![]()
![]() 4
4
��������
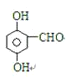
Aˮ�⡢�ữ�õ�B��C��D����A����������B��E��Ũ��������F��C3H6O2��ӦΪ������ת����ϵ��֪DΪȩ��EΪ����B����Է���������D��2����BΪCH3COOH��DΪHCHO��EΪCH3OH��FΪCH3COOCH3��C��FeCl3��Һ����ɫ�����з��ǻ������A�ķ���ʽ��֪C����7��Cԭ�ӣ�A�IJ����Ͷ�Ϊ=(2��10+2-9-1)/2=6������֪A�к���2��������������������γɵ�����������ˮ��õ���ȩ���ʴ���-COOCH2Cl�ṹ����C�л�����-COOH����C�ĺ˴Ź������������ĸ��壬������֮��Ϊ1��2��2��1����C�������������ڶ�λ����CΪ![]() ��AΪ
��AΪ![]() ��
��
��1��������������֪��BΪCH3COOH������ʽ��C2H4O2��
��2��DΪHCHO������D�й����ſ�ʹ���Լ�Ϊ��������Һ������Cu��OH��2����Һ��D������ͬϵ��ΪCH3CHO������Լ���Ӧ�Ļ�ѧ����ʽΪ��CH3CHO+2Ag��NH3��2OH![]() CH3COONH4+2Ag+3NH3��+H2O��CH3CHO+NaOH+2Cu��OH��2
CH3COONH4+2Ag+3NH3��+H2O��CH3CHO+NaOH+2Cu��OH��2![]() CH3COONa+Cu2O��+3H2O��
CH3COONa+Cu2O��+3H2O��
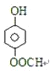
��3��������������֪��C�Ľṹ��ʽΪ��![]() ��
��
��4��C��![]() ����ͬ���칹����������������ٱ������ж�λȡ����������FeCl3��Һ����ɫ�����з��ǻ���������������Һ��Ӧ��˵������ȩ�������������ĵ���
����ͬ���칹����������������ٱ������ж�λȡ����������FeCl3��Һ����ɫ�����з��ǻ���������������Һ��Ӧ��˵������ȩ�������������ĵ��� ��
�� ��
�� ��
�� ��
��
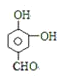
��5��������������֪��A�Ľṹ��ʽΪ��AΪ![]() ��
��
��6����1molA������NaOH��Ӧ��1molA��2mol����Ҫ����2mol NaOH����ClҪ����1mol NaOH��ˮ������1mol��Ҫ����1mol NaOH�����������4mol NaOH��