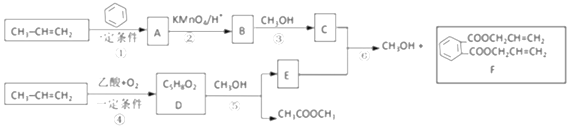
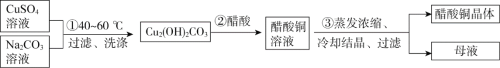
��Ŀ����
����Ŀ����������������㷺Ӧ��������������졢��������
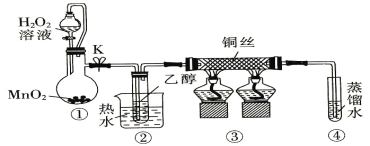
(1)����������ѭ���ֽ�ˮ��H2
��֪��H2O(l)===H2(g)��![]() O2(g)����H1����285.5 kJ/mol
O2(g)����H1����285.5 kJ/mol
6FeO(s)��O2(g) ===2Fe3O4(s)����H2����313.2 kJ/mol
��3FeO(s)��H2O(l)===H2(g)��Fe3O4(s)����H3��___________
(2)Fe2O3��CH4��Ӧ���Ʊ������������������䷴ӦΪ�� 3CH4(g) �� Fe2O3(s) ![]() 2Fe(s) ��6H2(g) ��3CO(g) ��H4
2Fe(s) ��6H2(g) ��3CO(g) ��H4
�ٴ˷�Ӧ�Ļ�ѧƽ�ⳣ������ʽΪ_________________________________��
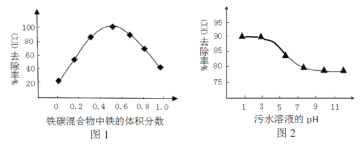
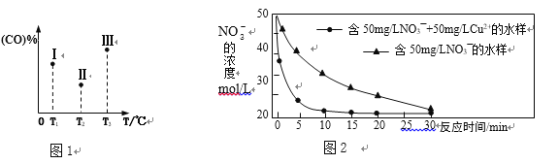
�����ݻ���ΪVL������������������ͬ�ܱ������м�����������������������Ȼ��ֱ����amolCO��2a molH2�����������ķ�Ӧ�¶ȷֱ𱣳�T1��T2��T3��������������ͬ������£�ʵ���÷�Ӧ�����е�tminʱCO�����������ͼ1��ʾ����ʱI��II��III����������һ�����ڻ�ѧƽ��״̬����___________(ѡ�����������������)���Ʊ����������������ķ�Ӧ����H4 _____ 0(���������)��
����T���£���ij�����ܱ������м���3molCH4(g)��2mol Fe2O3(s)����������Ӧ����Ӧ��ʼʱѹǿΪP0����Ӧ������10minʱ�ﵽƽ��״̬����ô�ʱ����������ѹǿ����ʼѹǿ��2����10 min����Fe2O3(s)��ʾ��ƽ����Ӧ����Ϊ_______g��min��1�� T���¸÷�Ӧ��Kp = _____________________��T��������ʼʱ��������м���2molCH4(g)��4mol Fe2O3(s)��1molFe(s)��2mol H2(g)��2molCO(g)������ʼʱv (��)______v (��) (������������������������)��
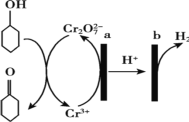
(3)����������ˮ��NO3����Ӧ�����ӷ���ʽΪ 4Fe+ NO3��+10H+=4Fe2++NH4++3H2O
���о����֣���pHƫ�ͽ��ᵼ��NO3����ȥ�����½�����ԭ����_________________��
����ͬ�����£���������ȥ����ͬˮ����NO3���������нϴ���죬ͼ2���������IJ���Ŀ���ԭ����__________________________________________________(��һ��)��
���𰸡���128.9 kJ/mol 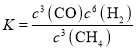 �� �� 8 P06 �� ����������H����Ӧ����H2 Cu��Cu2+����������ȥ��NO3���ķ�Ӧ(���γɵ�Fe��Cuԭ���������������ȥ��NO3���ķ�Ӧ����)
�� �� 8 P06 �� ����������H����Ӧ����H2 Cu��Cu2+����������ȥ��NO3���ķ�Ӧ(���γɵ�Fe��Cuԭ���������������ȥ��NO3���ķ�Ӧ����)
��������
(1)��������Ȼ�ѧ����ʽ����ϸ�˹���ɽ��м��㣻
(2)�ٻ�ѧƽ�ⳣ��=![]() ��
��
��2Fe(s)��6H2(g)��3CO(g)3CH4(g)��Fe2O3(s)������ͼ1������������ͼ��CO�ٷֺ�����С�����˳��Ϊ��������������ϻ�ѧƽ���ƶ�������𣻸����¶ȶ�ƽ���Ӱ�����жϣ������¶�ƽ�������ƶ���CO��ת���ʼ�С���ݴ��ж���H4��С��
����T���£���ij�����ܱ������м���3molCH4(g)��2mol Fe2O3(s)���з�Ӧ��3CH4(g)��Fe2O3(s)2Fe(s)��H2(g)��3CO(g)����Ӧ��ʼʱѹǿΪP0����Ӧ������10minʱ�ﵽƽ��״̬����ô�ʱ����������ѹǿ����ʼѹǿ��2�����г�����ʽ������Fe2O3(s)��ʾ��ƽ����Ӧ���ʣ�Kp��![]() ������ʼʱ��������м���2molCH4(g)��4mol Fe2O3(s)��1molFe(s)��2mol H2(g)��2molCO(g)������QC��K�Ĺ�ϵ�жϷ�Ӧ���еķ���
������ʼʱ��������м���2molCH4(g)��4mol Fe2O3(s)��1molFe(s)��2mol H2(g)��2molCO(g)������QC��K�Ĺ�ϵ�жϷ�Ӧ���еķ���
(3)��pHƫ�ͣ�������Ũ��ƫ���������������ӷ�Ӧ����������
����ͼ2��֪ͭ����Ũ��Խ��ȥ����Խ��ͭ���ӿ������ã�Ҳ�����γ�ԭ��ط�Ӧ��
(1)��֪����H2O(l)=H2(g)��![]() O2(g)����H1����285.5 kJ/mol����6FeO(s)��O2(g)=2Fe3O4(s)����H2����313.2 kJ/mol����3FeO(s)��H2O(l)=H2(g)��Fe3O4(s)����H3���ɸ�˹���ɿɵã���=��+
O2(g)����H1����285.5 kJ/mol����6FeO(s)��O2(g)=2Fe3O4(s)����H2����313.2 kJ/mol����3FeO(s)��H2O(l)=H2(g)��Fe3O4(s)����H3���ɸ�˹���ɿɵã���=��+![]() ���ڣ�����H3=��H1+
���ڣ�����H3=��H1+![]() ����H2=��285.5 kJ/mol+
����H2=��285.5 kJ/mol+![]() ��(��313.2 kJ/mol)=��128.9 kJ/mol��
��(��313.2 kJ/mol)=��128.9 kJ/mol��
(2)��3CH4(g)��Fe2O3(s)2Fe(s)��6H2(g)��3CO(g)�Ļ�ѧƽ�ⳣ������ʽΪ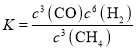 ��
��
�����ݻ���ΪVL������������������ͬ�ܱ������м�����������������������Ȼ��ֱ����a molCO��2a mol H2��������Ӧ2Fe(s)��6H2(g)��3CO(g)3CH4(g)��Fe2O3(s)������ͼ1������������ͼ��CO�ٷֺ�����С��������Ϊ��������������T1�е�״̬ת���T2�е�״̬��CO�ٷֺ�����С��˵��ƽ�������ƶ���˵��T1δ��ƽ��״̬��T2�е�״̬ת���T3�е�ƽ��״̬��CO�ٷֺ�������˵��ƽ�������ƶ���˵��T2���ܴ�ƽ��״̬��һ���ﵽ��ѧƽ��״̬��������2Fe(s)��6H2(g)��3CO(g)3CH4(g)��Fe2O3(s)���÷�Ӧ����ӦΪ���ȷ�Ӧ����������Ӧ3CH4(g)��Fe2O3(s)2Fe(s)��6H2(g)��3CO(g)����H4����0��
����T���£���ij�����ܱ������м���3molCH4(g)��2mol Fe2O3(s)����������Ӧ����Ӧ��ʼʱѹǿΪP0����Ӧ������10minʱ�ﵽƽ��״̬����ô�ʱ����������ѹǿ����ʼѹǿ��2���������ļ�������ʵ���Ϊxmol����
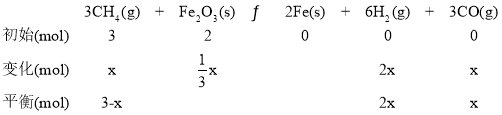
ѹǿ֮��Ϊ�������ʵ���֮�ȣ���ƽ��ʱ�������ʵ���Ϊ6mol����ʽΪ3x+2x+x��6�����x��1.5mol��10 min����Fe2O3(s)��ʾ��ƽ����Ӧ����Ϊ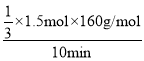 =8g/min��T���¸÷�Ӧ��Kp =
=8g/min��T���¸÷�Ӧ��Kp =![]() =
=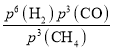 =
=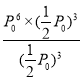 =P06���������������Ϊ1L������T���£�ƽ��ʱ��1.5molCH4(g)��3molH2(g)��1.5molCO(g)���÷�Ӧ��ƽ�ⳣ��K=
=P06���������������Ϊ1L������T���£�ƽ��ʱ��1.5molCH4(g)��3molH2(g)��1.5molCO(g)���÷�Ӧ��ƽ�ⳣ��K= ![]() =
=![]() =36������ʼʱ��������м���2molCH4(g)��4mol Fe2O3(s)��1molFe(s)��2mol H2(g)��2molCO(g)��QC=
=36������ʼʱ��������м���2molCH4(g)��4mol Fe2O3(s)��1molFe(s)��2mol H2(g)��2molCO(g)��QC= ![]() =
=![]() =26<K, ƽ�������ƶ�������ʼʱv (��)>v (��)��
=26<K, ƽ�������ƶ�������ʼʱv (��)>v (��)��
(3)��pHƫ�ͣ�������Ũ��ƫ���������������ӷ�Ӧ�����������ɵ���NO3-��ȥ�����½���
����ͼ2��֪ͭ����Ũ��Խ��ȥ����Խ��ͭ���ӿ������ã�Ҳ�����γ�ԭ��ط�Ӧ��

 ������ѧ��ʱ��ҵϵ�д�
������ѧ��ʱ��ҵϵ�д� ���������ʱ��ѵϵ�д�
���������ʱ��ѵϵ�д� �㽭�¿γ���άĿ�������ʱ��ѵϵ�д�
�㽭�¿γ���άĿ�������ʱ��ѵϵ�д� ��������ϵ�д�
��������ϵ�д�