��Ŀ����
����Ŀ����3mol/L NaOH��Һ���뵽25mL һ��Ũ�ȵ�AlCl3��Һ�С���ͼ���������̵���ѧ�������ߡ����к����ʾ����OH-�����ʵ����������ʾ������Al(OH)3���������ʵ������ͼ�ش�
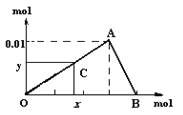
��1����C���O����A���˶�ʱ����ʾ��OH-�IJ��ϼ��룬��������________��OA���߱�ʾ�����ӷ�Ӧ��________________���ɴ˿ɵ�A�������Ϊ_______��
��2����C���A����B���˶�ʱ����ʾ��_____��AB���߱�ʾ�����ӷ�ӦΪ��______���ɴ˿ɵ�B�������Ϊ��__________��
��3�������������������AlCl3��Һ��Al3+���ӵ�Ũ��Ϊ��_____mol/L��
���𰸡����� Al3+ + 3OH�� = Al(OH)3�� ��0.03��0.01�� ����OH���IJ��ϼ��룬�����������ϼ��� Al(OH)3 + OH- = AlO2- + 2H2O (0.04��0) 0.4
��������
����ͼ��C���O����A���˶�ʱ����ʾ����OH-�IJ��ϼ��룬�����������ӣ�A��Al3+�պ���ȫ�����������������ֵ����A�㵽B�㣬����OH-�IJ��ϼ��룬���������٣�B��ʱAl(OH)3�պ���ȫ�ܽ⡣����������n[Al(OH)3]max=0.01mol��Al�غ㣬ԭn��AlCl3��=0.01mol��
��1����C���O����A���˶�ʱ����ʾ����OH-�IJ��ϼ��룬�����������ӣ�OA���߱�ʾ�����ӷ�Ӧ�ǣ�Al3++3OH-= Al(OH)3������A�����ĵ�n��OH-��=3![]() 0.01mol=0.03mol���ɴ˿ɵ�A�������Ϊ��0.03��0.01����
0.01mol=0.03mol���ɴ˿ɵ�A�������Ϊ��0.03��0.01����
��2����C���A����B���˶�ʱ����ʾ������OH���IJ��ϼ��룬�����������ϼ��٣�AB���߱�ʾAl(OH)3����NaOH����ʾ�����ӷ�ӦΪAl(OH)3 + OH- = AlO2- + 2H2O���ܽ�0.01mol Al(OH)3����n��OH-��=0.01mol����B�������Ϊ��0.04��0����
��3��ԭn��AlCl3��=0.01mol��c��AlCl3��=![]() =0.4mol/L��AlCl3��Һ��Al3+���ӵ�Ũ��Ϊ0.4mol/L��
=0.4mol/L��AlCl3��Һ��Al3+���ӵ�Ũ��Ϊ0.4mol/L��

 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�