��Ŀ����
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������___________________________________��
(2)���м�������������ɫ��������ӷ���ʽ��________________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�________�ԣ�ԭ����____________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ____________________________����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
| ������ | K����Ag����Mg2����Cu2����Al3����NH4+ |
| ������ | Cl����CO32����NO3����SO42����I�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������___________________________________��
(2)���м�������������ɫ��������ӷ���ʽ��________________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�________�ԣ�ԭ����____________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ____________________________����ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
(1)K����NH4+��Cu2��
(2)6I����2NO3����8H��=3I2��2NO����4H2O
(3)Mg2����Al3����Cl����NO3����SO42����I�����ᡡMg2����2H2O??Mg(OH)2��2H����Al3����3H2O Al(OH)3��3H��
Al(OH)3��3H��
(4)Mg2����2OH��=Mg(OH) 2����Al3����4OH��=AlO2����2H2O��0.4
(2)6I����2NO3����8H��=3I2��2NO����4H2O
(3)Mg2����Al3����Cl����NO3����SO42����I�����ᡡMg2����2H2O??Mg(OH)2��2H����Al3����3H2O
 Al(OH)3��3H��
Al(OH)3��3H��(4)Mg2����2OH��=Mg(OH) 2����Al3����4OH��=AlO2����2H2O��0.4
(1)���ݢ���ҺΪ��ɫ������Һ�в���Cu2�������ɳ����������������ӣ�˵��ԭ��Һ�в���NH4+�����ݢ��ȷ����Һ�в���K����(2)���ݢ��������ȷ�����ɵ���ɫ����ΪNO����ԭ��Һ��һ������NO3����I��������ת�Ƶ�����Ŀ��ȡ�����غ��д�����ӷ���ʽ��(3)��Ϣ�����Һ�и�����Ũ����ȿ�ȷ����Һ�к��е�������ΪSO42����Cl����NO3������Һ�����ԣ���Mg2����Al3��ˮ���Ե�ʡ�(4)����NaOH��Һ��Mg2��ת��ΪMg(OH)2������Al3�����ת��ΪNaAlO2�����յõ�0.01 mol Mg(OH)2���������������صõ�0.4 g MgO��
�㲦�������Ϸ�ˮ�ijɷֿ������Ӽ�������ӷ���ʽ����д�����ڿ��鿼���ۺϷ�������������ͼ���������
�㲦�������Ϸ�ˮ�ijɷֿ������Ӽ�������ӷ���ʽ����д�����ڿ��鿼���ۺϷ�������������ͼ���������

��ϰ��ϵ�д�
�����Ŀ
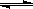 2H++Cl��+ClO��
2H++Cl��+ClO��