��Ŀ����
����Ŀ���л���D�������Ժ�ǿ�����ԣ���һ�ָ�Ч����ɱ���������Ʊ�·�����£�
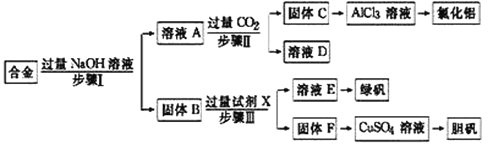
��֪��A�ڱ���µ��ܶ�Ϊ1.25gL-1��B�������ԣ�C�ܷ���������Ӧ����ش�
��1��A�Ľṹ��ʽ______��
��2��C��D�ķ�Ӧ����______��
��3��B��D�Ļ�ѧ����ʽ______��
��4������˵������ȷ����______��
A����ʯ�͵õ�A�ķ�����Ϊ�ѻ�
B����A��һ������������B�����ӳɷ�Ӧ������������Ϊ��������
C���л���D��ɱ����������Ҫԭ���Ǻ���-COOH�ṹ
D��C��ʹ�������������Һ����ˮ��ɫ����ԭ����ͬ
���𰸡�CH2=CH2 ������Ӧ CH3COOH+H2O2��CH3COOOH+H2O AC
��������
A�ڱ�״���µ��ܶ�Ϊ1.25gL-1����A��Ħ������Ϊ1.25g/L��22.4L/mol=28g/mol��A��Դ��ʯ�ͣ���AΪCH2=CH2��������ͼ��֪��A��������B��B�������ԣ���BΪCH3COOH��A��������CΪCH3CHO��B��������ⷢ��������Ӧ����DΪCH3COOOH��C����������������Ӧ����D��
��1��������������֪��A�Ľṹ��ʽΪCH2=CH2���ʴ�Ϊ��CH2=CH2��
��2����C��D�ķ���ʽ��֪��Oԭ�������࣬C��D �ķ�Ӧ����Ϊ������Ӧ���ʴ�Ϊ��������Ӧ��
��3��B��D�Ļ�ѧ����ʽΪCH3COOH+H2O2��CH3COOOH+H2O���ʴ�Ϊ��CH3COOH+H2O2��CH3COOOH+H2O��
��4��A����ʯ�͵õ�A �ķ�����Ϊ���ʹ����ת��Ϊ������С���ӣ���Ϊ�ѽ⣬��A����
B���� A ��һ������������B�����ӳɷ�Ӧ���������ΪCH3CH2OOCCH3��Ϊ������������B��ȷ��
C���л��� D ��ɱ����������Ҫԭ���Ǻ���-O-O-�ṹ������ǿ�����ԣ���C����
D��CΪ��ȩ����-CHO����ʹ�������������Һ����ˮ��ɫ����ԭ����ͬ����Ϊ������Ӧ����D��ȷ���ʴ�Ϊ��AC��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ�����й������ӷ���ʽ��������ȷ����
ѡ�� | ���ӷ���ʽ | ���� |
A | ��1molCl2ͨ�뵽��2molFeI2����Һ�У�2Fe2����Cl2==2Fe3��+2Cl�� | ��ȷ��Fe2���Ļ�ԭ��ǿ��I�� |
B | �ö��Ե缫���MgCl2��Һ:2Cl-+2H2O | ����Ӧ����Mg(OH)2���� |
C | ����SO2ͨ�뵽NaClO��Һ�У�SO2��H2O+ClO��===HClO+HSO3�� | ��ȷ��H2SO3������ǿ��HClO |
D | Mg(HCO3)2��Һ��������NaOH��Һ��Ӧ��Mg2��+2HCO3��+2OH��===MgCO3��+2H2O | ��ȷ��MgCO3��Mg(OH)2������ |
A.AB.BC.CD.D